| |
| Names | |
|---|---|
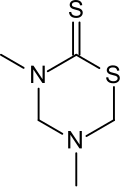
| Preferred IUPAC name
3,5-Dimethyl-1,3,5-thiadiazinane-2-thione | |
| Other names
Mylon; Basamid, Thiazone; Mylone; Tiazon; DMTT; Dimethylformocarbothialdine; Carbothialdin; Basamide; Nefusan; Prezervit
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.798 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H10N2S2 | |
| Molar mass | 162.27 g·mol−1 |
| Appearance | White solid |
| Density | 1.29±0.1 g/mL[1] |
| Melting point | 104 to 105 °C (219 to 221 °F; 377 to 378 K) |
| Boiling point | 222.3±50.0 °C (predicted)[1] |
| Hazards | |
| Flash point | 156 °C (313 °F)[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Dazomet is a common soil fumigant that acts as a herbicide, fungicide, slimicide,[3] and nematicide.
YouTube Encyclopedic
-
1/3Views:3 737601608
-
Cuidado y Tratamiento de Cítricos en Vivero - TvAgro por Juan Gonzalo Angel
-
Malagueña
-
Micorrizas Arbusculares em Plantas Frutíferas Tropicais
Transcription
Applications
Dazomet is a chemical used to kill pests that inhibit plant growth through gaseous degradation. Dazomet is used as a soil sterilant on a variety of sites such as golf courses, nurseries, turf sites, and potting soils.[4] Dazomet is used for soil sterilization as an alternative to methyl bromide. Although less effective it is still used to kill pests because of its comparatively lower toxicity. Dazomet is applied to wet soil, which causes dazomet itself to decompose into a gaseous form, which is what actively controls pests. The decomposition of dazomet releases methyl isothiocyanate (MITC) a gas toxic to pests that would prevent or kill plant growth. Dazomet has also been proven to be effective in the prevention of Phellinus noxius, or brown root rot disease.[5]
Synthesis
Dazomet is synthesized from carbon disulfide (CS2) and diluted methylamine (CH3NH2). After stirring for 1-2 hours, an oily substance is formed, which is the intermediate methyldithiocarbamic acid (HS2CNHCH3). Then, formaldehyde (CH2O) is added to the intermediate to form and precipitate out the dazomet.[6]
Form, color, and smell
Dazomet takes the form of colorless crystals.[7][8] Dazomet is described as having a weakly pungent[9] smell.
Toxicology and safety
Dazomet is irritating to the eyes[10] and its degradation product, MITC, is a dermal sensitizer. Dazomet is very toxic to aquatic organisms, and also acutely toxic to mammals. Exposure to dazomet can occur through several means; interaction with unincorporated granules, inhalation of it decomposition product, MITC, and/or water runoff.
Stability and shelf life
Dazomet is stable at temperatures up to 35°C; it is sensitive to temperature >50°C and to moisture.[7][11] Dazomet has a shelf life of at least two years when stored below 50°C.[12]
Mass spectrum
The molecular ion peak of dazomet is at 162 m/z. There are two major fragment peaks, one at 89 m/z and one at 42 m/z. The fragment peak at 89 m/z represents the loss of MITC, which is the major, gaseous degrade of dazomet.[13]
Patents
- Patent #US5989597
- Ambrose Rajamannan was the inventor of the novel process of continuously and instantaneously sterilizing soil using a water-activated fumigant. Dazomet is one water-activated fumigant that could be used. This process ensures 100% activation of the fumigant. Therefore, less fumigant would be needed to ensure that the soil would be completely sterilized.[14]
- Patent #CN1543787
- See under § Synthesis
References
- ^ a b SciFinder.Dazomet.properties (accessed Oct. 5, 2014).
- ^ "MSDS for Diazomet". Sigma-Aldrich.
- ^ "Revised Environmental ate and Ecological Risk Assessment for Dazomet" (https://www3.epa.gov/pesticides/chem_search/cleared_reviews/csr_PC-035602_08-Apr-08_a.pdf) (PDF) US Environmental Protection Agency, Office of Prevention, Pesticides, and Toxic Substances. April 8, 2008.
- ^ "RED Fact Sheet: Dazomet" (PDF). US Environmental Protection Agency, Office of Pesticide Programs. July 10, 2008. Archived from the original (PDF) on 2014-10-16.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Fu, C.; Hu, B.; Chang, T.; Hsueh, K.; Hsu, W. Evaluation of dazomet as fumigant for the control of brown root rot disease. Pest Management Science 2012, 68, 959-962.
- ^ Cao, Y.; Lu, Y. Process for preparing dazomet dustless stable fine granules. CN1543787, Nov. 10, 2004.
- ^ a b "Dazomet". PubChem. National Center for Biotechnology Information. Retrieved 2021-11-12.
- ^ Tomlin CDS, ed; The e-Pesticide Manual: a world compendium. Dazomet. 13th ed. PC CD-ROM, Version 3.0, 2003-04. Surrey, UK: British Crop Protection Council, (2003)
- ^ Meister, R.T., Sine, C. (eds) Crop Protection Handbook Volume 92, Willoughby, OH, 2006., p. D 127
- ^ "Harmonised classification of dazomet in the C&L Inventory". ECHA. Retrieved 2019-02-11.
- ^ Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium. 10th ed. Surrey, UK: The British Crop Protection Council, 1994., p. 280
- ^ Hartley, D. and H. Kidd (eds.). The Agrochemicals Handbook. Old Woking, Surrey, United Kingdom: Royal Society of Chemistry/Unwin Brothers Ltd., 1983., p. A114/Oct 83
- ^ NIST Chemistry WebBook. 2H-1,3,5-Thiadiazine-2-thione, tetrahydro-3,5-dimethyl-. http://webbook.nist.gov/ (accessed Oct. 5, 2014).
- ^ Rajamannan, A. H. J. Process for sterilizing soil. US Patent 5989597, Nov. 23, 1999.
