
The NH3 and Cl groups form a coordination sphere around the central cobalt ion.
In coordination chemistry, the first coordination sphere refers to the array of molecules and ions (the ligands) directly attached to the central metal atom. The second coordination sphere consists of molecules and ions that attached in various ways to the first coordination sphere.
YouTube Encyclopedic
-
1/3Views:113 68910 51015 501
-
Coordination Compounds: Geometry and Nomenclature
-
Coordination number | coordination sphere | Part 12 | Unit 9| CBSE grade 12 chemistry.
-
21.3 Isomers in Coordination Chemistry | General Chemistry
Transcription
First coordination sphere
The first coordination sphere refers to the molecules that are attached directly to the metal. The interactions between the first and second coordination spheres usually involve hydrogen-bonding. For charged complexes, ion pairing is important.
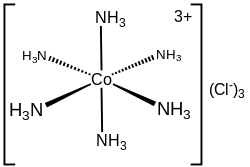
In hexamminecobalt(III) chloride ([Co(NH3)6]Cl3), the cobalt cation plus the 6 ammonia ligands comprise the first coordination sphere. The coordination sphere of this ion thus consists of a central MN6 core "decorated" by 18 N−H bonds that radiate outwards.
Second coordination sphere

Metal ions can be described as consisting of series of two concentric coordination spheres, the first and second. More distant from the second coordination sphere, the solvent molecules behave more like "bulk solvent." Simulation of the second coordination sphere is of interest in computational chemistry. The second coordination sphere can consist of ions (especially in charged complexes), molecules (especially those that hydrogen bond to ligands in the first coordination sphere) and portions of a ligand backbone. Compared to the first coordination sphere, the second coordination sphere has a less direct influence on the reactivity and chemical properties of the metal complex. Nonetheless the second coordination sphere is relevant to understanding reactions of the metal complex, including the mechanisms of ligand exchange and catalysis.
Role in catalysis
Mechanisms of metalloproteins often invoke modulation of the second coordination sphere by the protein.[1]

Role in mechanistic inorganic chemistry
The rates at which ligands exchange between the first and the second coordination sphere is the first step in ligand substitution reactions. In associative ligand substitution, the entering nucleophile resides in the second coordination sphere. These effects are relevant to practical applications such as contrast agents used in MRI.[4]
The energetics of inner sphere electron transfer reactions are discussed in terms of second coordination sphere. Some proton coupled electron transfer reactions involve atom transfer between the second coordination spheres of the reactants:
- [Fe*(H2O)6]2+ + [Fe(H2O)5(OH)]2+ → [Fe(H2O)6]3+ + [Fe*(H2O)5(OH)]2+
Role in spectroscopy
Solvent effects on colors and stability are often attributable to changes in the second coordination sphere. Such effects can be pronounced in complexes where the ligands in the first coordination sphere are strong hydrogen-bond donors and acceptors, e.g. respectively [Co(NH3)6]3+ and [Fe(CN)6]3−. Crown-ethers bind to polyamine complexes through their second coordination sphere. Polyammonium cations bind to the nitrogen centres of cyanometallates.[5]
Role in supramolecular chemistry
Macrocyclic molecules such as cyclodextrins act often as the second coordination sphere for metal complexes. [6][7]
See also
Further reading
References
- ^ Zhao, Meng; Wang, Hai-Bo; Ji, Liang-Nian; Mao, Zong-Wan (2013). "Insights into metalloenzyme microenvironments: biomimetic metal complexes with a functional second coordination sphere". Chemical Society Reviews. 42 (21): 8360–8375. doi:10.1039/c3cs60162e. ISSN 0306-0012. PMID 23881282.
- ^ Yang, J. Y.; Chen, S.; Dougherty, W. G.; Kassel, W. S.; Bullock, R. M.; DuBois, D. L.; Raugei, S.; Rousseau, R.; Dupuis, M.; Rakowski DuBois, M. (2010). "Hydrogen oxidation catalysis by a nickel diphosphine complex with pendant tert-butyl amines". Chem. Commun. 46 (45): 8618–8620. doi:10.1039/c0cc03246h. PMID 20938535.
- ^ Bullock, R. M.; Helm, M. L. (2015). "Molecular Electrocatalysts for Oxidation of Hydrogen Using Earth-Abundant Metals: Shoving Protons Around with Proton Relays". Acc. Chem. Res. 48 (7): 2017–2026. doi:10.1021/acs.accounts.5b00069. OSTI 1582563. PMID 26079983.
- ^ R. M. Supkowski, W. DeW. Horrocks Jr. "On the determination of the number of water molecules, q, coordinated to europium(III) ions in solution from luminescence decay lifetimes" Inorganic Chimica Acta 2002, Volume 340, pp. 44–48. doi:10.1016/S0020-1693(02)01022-8
- ^ Lehn, J. M. Supramolecular Chemistry: Concepts and Perspectives; VCH: Weinhiem, 1995.
- ^ Z. Liu, S. T. Schneebeli, J. F. Stoddart "Second-sphere coordination revisited" Chimia 2014, 68, 315-320. doi:10.2533/chimia.2014.315
- ^ Z. Liu, M. Frasconi, J. Lei, Z. J. Brown, Z. Zhu, D. Cao, J. Iehl, G. Liu, A. C. Fahrenbach, O. K. Farha, J. T. Hupp, C. A. Mirkin, Y. Y. Botros, J. F. Stoddart "Selective isolation of gold facilitated by second-sphere coordination with alpha-cyclodextrin" Nature Communications 2013, 4, 1855. doi:10.1038/ncomms2891
