In organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water.[1] If water is lost, the reaction is also known as a dehydration synthesis. However other molecules can also be lost, such as ammonia, ethanol, acetic acid and hydrogen sulfide.[2]
The addition of the two molecules typically proceeds in a step-wise fashion to the addition product, usually in equilibrium, and with loss of a water molecule (hence the name condensation).[3] The reaction may otherwise involve the functional groups of the molecule, and is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and to the biosynthesis of fatty acids.[4]
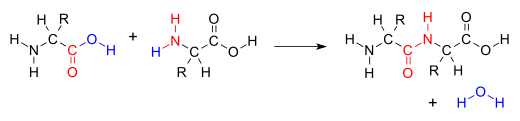
Many variations of condensation reactions exist. Common examples include the aldol condensation and the Knoevenagel condensation, which both form water as a by-product, as well as the Claisen condensation and the Dieckman condensation (intramolecular Claisen condensation), which form alcohols as by-products.[5]
YouTube Encyclopedic
-
1/3Views:5 01210 447229 923
-
What are condensation reactions? | A-Level Biology
-
Condensation Reactions
-
Aldol Condensation
Transcription
Synthesis of prebiotic molecules
Condensation reactions likely played major roles in the synthesis of the first biotic molecules including early peptides and nucleic acids. In fact, condensation reactions would be required at multiple steps in RNA oligomerization: the condensation of nucleobases and sugars, nucleoside phosphorylation, and nucleotide polymerization.[6]
See also
- Anabolism
- Hydrolysis, the opposite of a condensation reaction
- Condensed tannins
References
- ^ "25.18 Condensation Reactions". Book: Introductory Chemistry (CK-12). Chemistry Libre Texts. 12 August 2020. Retrieved 9 January 2021.
- ^ "Condensation Reaction". IUPAC Compendium of Chemical Terminology (Gold Book). IUPAC. 2014. doi:10.1351/goldbook.C01238. Retrieved 7 December 2017.
- ^ Fakirov, S. (2019-02-01). "Condensation Polymers: Their Chemical Peculiarities Offer Great Opportunities". Progress in Polymer Science. 89: 1–18. doi:10.1016/j.progpolymsci.2018.09.003. ISSN 0079-6700. S2CID 105101288.
- ^ Voet, Donald; Voet, Judith; Pratt, Chriss (2008). Fundamentals of Biochemistry. Hoboken, NJ: John Wiley & Sons, Inc. pp. 88. ISBN 978-0470-12930-2.
- ^ Bruckner, Reinhard (2002). Advanced Organic Chemistry (First ed.). San Diego, California: Harcourt Academic Press. pp. 414–427. ISBN 0-12-138110-2.
- ^ Fiore, Michele (2022). Prebiotic Chemistry and Life's Origin. United Kingdom: Royal Society of Chemistry. pp. 124–144. ISBN 9781839164804.

