 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.220.474 |
| Chemical and physical data | |
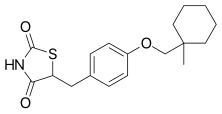
| Formula | C18H23NO3S |
| Molar mass | 333.45 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ciglitazone (INN) is a thiazolidinedione. Developed by Takeda Pharmaceuticals in the early 1980s, it is considered the prototypical compound for the thiazolidinedione class.[1][2][3][4]
Ciglitazone was never used as a medication, but it sparked interest in the effects of thiazolidinediones. Several analogues were later developed, some of which—such as pioglitazone and troglitazone—made it to the market.[2]
Ciglitazone significantly decreases VEGF production by human granulosa cells in an in vitro study, and may potentially be used in ovarian hyperstimulation syndrome.[5] Ciglitazone is a potent and selective PPARγ ligand. It binds to the PPARγ ligand-binding domain with an EC50 of 3.0 μM. Ciglitazone is active in vivo as an anti-hyperglycemic agent in the ob/ob murine model.[6] Inhibits HUVEC differentiation and angiogenesis and also stimulates adipogenesis and decreases osteoblastogenesis in human mesenchymal stem cells.[7]
References
- ^ Pershadsingh HA, Szollosi J, Benson S, Hyun WC, Feuerstein BG, Kurtz TW (June 1993). "Effects of ciglitazone on blood pressure and intracellular calcium metabolism". Hypertension. 21 (6 Pt 2): 1020–1023. doi:10.1161/01.hyp.21.6.1020. PMID 8505086.
- ^ a b Hulin B, McCarthy PA, Gibbs EM (1996). "The glitazone family of antidiabetic agents". Current Pharmaceutical Design. 2: 85–102. doi:10.2174/1381612802666220920215821. S2CID 252485570.
- ^ Imoto H, Imamiya E, Momose Y, Sugiyama Y, Kimura H, Sohda T (October 2002). "Studies on non-thiazolidinedione antidiabetic agents. 1. Discovery of novel oxyiminoacetic acid derivatives". Chemical & Pharmaceutical Bulletin. 50 (10): 1349–1357. doi:10.1248/cpb.50.1349. PMID 12372861.
- ^ Sohda T, Kawamatsu Y, Fujita T, Meguro K, Ikeda H (November 2002). "[Discovery and development of a new insulin sensitizing agent, pioglitazone]". Yakugaku Zasshi (in Japanese). 122 (11): 909–918. doi:10.1248/yakushi.122.909. PMID 12440149.
- ^ Shah DK, Menon KM, Cabrera LM, Vahratian A, Kavoussi SK, Lebovic DI (April 2010). "Thiazolidinediones decrease vascular endothelial growth factor (VEGF) production by human luteinized granulosa cells in vitro". Fertility and Sterility. 93 (6): 2042–2047. doi:10.1016/j.fertnstert.2009.02.059. PMC 2847675. PMID 19342033.
- ^ Willson TM, Cobb JE, Cowan DJ, Wiethe RW, Correa ID, Prakash SR, et al. (February 1996). "The structure-activity relationship between peroxisome proliferator-activated receptor gamma agonism and the antihyperglycemic activity of thiazolidinediones". Journal of Medicinal Chemistry. 39 (3): 665–668. doi:10.1021/jm950395a. PMID 8576907.
- ^ Xin X, Yang S, Kowalski J, Gerritsen ME (March 1999). "Peroxisome proliferator-activated receptor gamma ligands are potent inhibitors of angiogenesis in vitro and in vivo". The Journal of Biological Chemistry. 274 (13): 9116–9121. doi:10.1074/jbc.274.13.9116. PMID 10085162.
