| |
| Names | |
|---|---|
| Other names
chlorine dioxide fluoride
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| ClFO2 | |
| Molar mass | 86.45 g·mol−1 |
| Density | 3.534 g/L |
| Melting point | −115 °C |
| Boiling point | −6 °C |
| Related compounds | |
Related compounds
|
Perchloryl fluoride Chloryl trifluoride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chloryl fluoride is the chemical compound with the formula ClO2F. It is commonly encountered as side-product in reactions of chlorine fluorides with oxygen sources.[1] It is the acyl fluoride of chloric acid.
YouTube Encyclopedic
-
1/5Views:1 095 38032 993 8877 053 24719 323 433170 842
-
Setting Fire to Glass - The "Nope" Chemical That is Chlorine Trifluoride
-
Mixing sodium with mercury
-
Cesium is scary
-
Calcium is crazy
-
How to make a Stannous Chloride Solution (Tin (II) Chloride)
Transcription
Preparation
ClO2F was first reported by Schmitz and Schumacher in 1942, who prepared it by the fluorination of ClO2.[2] The compound is more conveniently prepared by reaction of sodium chlorate and chlorine trifluoride[3] and purified by vacuum fractionation, i.e. selectively condensing this species separately from other products. This species is a gas boiling at −6 °C:
- 6 NaClO3 + 4 ClF3 → 6 ClO2F + 2 Cl2 + 3 O2 + 6 NaF
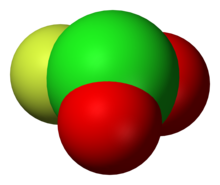
Structure
In contrast to O2F2, ClO2F is a pyramidal molecule. This structure is predicted by VSEPR. The differing structures reflects the greater tendency of chlorine to exist in positive oxidation states with oxygen and fluorine ligands. The related Cl-O-F compound perchloryl fluoride, ClO3F, is tetrahedral. The related bromine compound bromyl fluoride (BrO2F) adopts the same structure as ClO2F, whereas iodyl fluoride (IO2F) forms a polymeric substance under standard conditions.[4]
References
- ^ Chrisie, K. O.; Wilson, R. D.; Schack, C. J. "Chloryl fluoride" Inorganic Syntheses, 1986, volume 24, pages 3–5. ISBN 0-471-83441-6
- ^ Schmitz, H.; Schumacheb, H. J. (1942-04-29). "Über eine neue Reaktion des Chlordioxyds. Die Bildung einer Verbindung der Formel ClO2F". Zeitschrift für anorganische und allgemeine Chemie (in German). Wiley. 249 (3): 238–244. doi:10.1002/zaac.19422490302. ISSN 0863-1786.
- ^ Wiberg, Egon; Wiberg, Nils; Holleman, A. F. (2001). Inorganic chemistry. San Diego: Academic Press. p. 1797. ISBN 0-12-352651-5. OCLC 48056955.
- ^ Holleman, A.F.; Wiberg, E.; Wiberg, N. (1995). Lehrbuch der anorganischen Chemie. de Gruyter. p. 501. ISBN 9783110126419. Retrieved 2015-02-20.
