| |
| Names | |
|---|---|
| IUPAC name
Cesium sulfide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Cs2S | |
| Molar mass | 297.876 g/mol |
| Appearance | White crystal |
| Density | 4.19 g·cm−3[1] |
| Melting point | 480 °C[2] |
| Hydrolyzes to form caesium bisulfide[3] | |
| Solubility in ethanol and glycerol | Soluble |
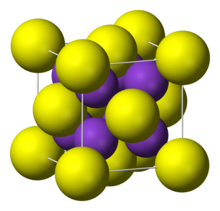
| Structure | |
| cubic, anti-fluorite | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
toxic |
| GHS labelling: | |
 
| |
| Danger | |
| H314, H400 | |
| P260, P264, P273, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P391, P405, P501 | |
| Related compounds | |
Other anions
|
Caesium oxide Caesium selenide Caesium telluride Caesium polonide |
Other cations
|
Lithium sulfide Sodium sulfide Potassium sulfide Rubidium sulfide Francium sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cesium sulfide is an inorganic salt with a chemical formula Cs2S. It is a strong alkali in aqueous solution. In the air, cesium sulfide emits rotten egg smelling hydrogen sulfide.
YouTube Encyclopedic
-
1/5Views:16 717 6501 8882 7876 343 7529 968 127
-
Cesium - The most ACTIVE metal on EARTH!
-
Lewis Structure of Cs2S, caesium sulfide
-
How to Write the Formula for Cesium sulfide
-
This chemical really doesn't want to exist
-
5 of the World's Most Dangerous Chemicals
Transcription
Production
Similar to sodium sulfide, anhydrous cesium sulfide can be produced by reacting cesium and sulfur in THF. It needs ammonia or naphthalene to react.[4]
- 2 Cs + S → Cs2S
By dissolving hydrogen sulfide into cesium hydroxide solution, it will produce cesium bisulfide, then it will produce cesium sulfide too.[5][6]。
- CsOH + H2S → CsHS + H2O
- CsHS + CsOH → Cs2S + H2O
References
- ^ Sommer, Helmut; Hoppe, Rudolf. The crystal structure of cesium sulfide and a remark about cesium selenide, cesium telluride, rubidium selenide, and rubidium telluride (in German). Zeitschrift für Anorganische und Allgemeine Chemie, 1977. 429: 118-30. ISSN 0044-2313
- ^ Dale L. Perry, Sidney L. Phillips: Handbook of inorganic compounds. CRC Press, 1995, ISBN 978-0-8493-8671-8, S. 336 ([1], p. 336, at Google Books).
- ^ Jean D'Ans, Ellen Lax: Taschenbuch für Chemiker und Physiker. 3. Elemente, anorganische Verbindungen und Materialien, Minerale, Band 3. 4. Auflage, Springer, 1997, ISBN 978-3-5406-0035-0, S. 692 ([2], p. 692, at Google Books).
- ^ J.-H. So and P. Boudjouk (1992). N. G. Russell (ed.). "Hexamethyldisilathiane". Inorganic Syntheses. 29: 30–32. doi:10.1002/9780470132609.ch11. ISBN 978-0-470-13260-9.
- ^ Biltz, Wilhelm; Wilke-Dörfurt, Ernst (1905). "Über Sulfide des Rubidiums und Cäsiums". Zeitschrift für Anorganische Chemie. 48: 297–318. doi:10.1002/zaac.19060480122.
- ^ R. Abegg, F. Auerbach: 'Handbuch der anorganischen Chemie'. Verlag S. Hirzel, Bd. 2, 1908. S. 430.Volltext
