 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
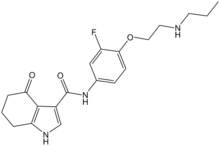
| Formula | C20H24FN3O3 |
| Molar mass | 373.428 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
CP-615,003 is a drug which acts as a subtype-selective partial agonist at GABAA receptors, and was developed by Pfizer as a potential anxiolytic; however, poor blood–brain barrier penetration make it primarily useful as a research ligand.[1][2]
References
- ^ Shaffer CL, Gunduz M, O'Connell TN, Obach RS, Yee S (November 2005). "Biotransformation of a GABAA receptor partial agonist in sprague-dawley rats and cynomolgus monkeys: identification of two unique N-carbamoyl metabolites". Drug Metabolism and Disposition. 33 (11): 1688–99. doi:10.1124/dmd.105.004630. PMID 16081672. S2CID 6983318.
- ^ Venkatakrishnan K, Tseng E, Nelson FR, Rollema H, French JL, Kaplan IV, Horner WE, Gibbs MA (August 2007). "Central nervous system pharmacokinetics of the Mdr1 P-glycoprotein substrate CP-615,003: intersite differences and implications for human receptor occupancy projections from cerebrospinal fluid exposures". Drug Metabolism and Disposition. 35 (8): 1341–9. doi:10.1124/dmd.106.013953. PMID 17470526. S2CID 32926420.
