Chemical compound
 | |
| Clinical data | |
|---|---|
| Other names | Brophebarbital |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
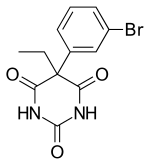
| Formula | C12H11BrN2O3 |
| Molar mass | 311.135 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Brophebarbital is a barbiturate derivative. It has sedative and hypnotic effects and is considered to have a moderate abuse potential.[1]
References
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids |
|
| Imidazoles | |
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
