
| |
| Names | |
|---|---|
| Preferred IUPAC name
(10E,12Z)-Hexadeca-10,12-dien-1-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C16H30O | |
| Molar mass | 238.415 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bombykol is a pheromone released by the female silkworm moth to attract mates. It is also the sex pheromone in the wild silk moth (Bombyx mandarina).[1][2] Discovered by Adolf Butenandt in 1959, it was the first pheromone to be characterized chemically.[3]
Minute quantities of this pheromone can be used per acre of land to confuse male insects about the location of their female partners. It can thus serve as a lure in traps to remove insects effectively without spraying crops with large amounts of pesticides. Butenandt named the substance after the moth's Latin name Bombyx mori.[4]
In vivo it appears that bombykol is the natural ligand for a pheromone binding protein, BmorPBP, which escorts the pheromone to the pheromone receptor.[5]
YouTube Encyclopedic
-
1/3Views:19 750568 9804 777
-
Pheromone
-
Do Humans Have Pheromones?
-
Divine Abodes: Equanimity - Tara Brach
Transcription
Biosynthesis
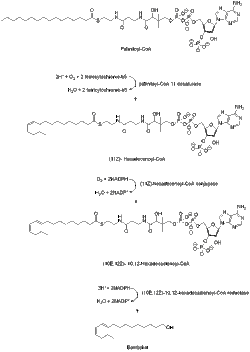
Bombykol is known to be derived from acetyl-CoA via the C-16 fatty acyl palmitoyl-CoA.[6] Palmitoyl-CoA is converted to bombykol in steps that involve desaturation and reductive modification of the carbonyl carbon.[7] Compared to other Type I pheromones, bombykol biosynthesis does not need chain-shortening or any other kind of modification of the terminal hydroxyl group.
A desaturase enzyme encoded by the gene Bmpgdesat1 (Desat1), produces the monoene (11Z)-hexadecenoyl-CoA as well as the diene (10E,12Z)-10,12-hexadecadienoyl-CoA. This desaturase is the only enzyme necessary to catalyze these two consecutive desaturation steps.[8]
The bombykol acyl precursor (10E,12Z)-10,12-hexadecadienoate is primarily found as a triacylglycerol ester in the cytoplasmic lipid droplets of pheromone gland cells of the moth. And when the adult females emerge from their pupae, the neurohormone PBAN (pheromone biosynthesis-activating neuropeptide) start signaling events that help control the lipolysis of the stored triacylglycerols, releasing (10E,12Z)-10,12-hexadecadienoate for its final reductive modification.[9][10][11] The mechanism of the lipolytic release of (10E,12Z)-10,12-hexadecadienoate from triacylglycerols is not completely known but the candidate lipase-encoding genes have been identified.[9][10]
References
- ^ Kuwahara, Yasumasa (1984). "Flight Time of Bombyx mandarina Males to a Pheromone Trap Baited with Bombykol". Applied Entomology and Zoology. 19 (3): 400–401. doi:10.1303/aez.19.400.
- ^ Kuwahara, Yasumasa; Mori, Naoki; Yamada, Shigeki; Nemoto, Tadashi (1984). "Evaluation of Bombykol as the Sex Pheromone of Bombyx mandarina(Lepidoptera : Bombycidae)". Applied Entomology and Zoology. 19 (2): 265–267. doi:10.1303/aez.19.265.
- ^ Butenandt, A.; Beckamnn, R.; Hecker, E. (1961). "Über den Sexuallockstoff des Seidenspinners .1. Der biologische Test und die Isolierung des reinen Sexuallockstoffes Bombykol". Hoppe-Seyler's Zeitschrift für Physiologische Chemie. 324: 71–83. doi:10.1515/bchm2.1961.324.1.71. PMID 13689417.
- ^ "Molecule of the Week". American Chemical Society. 2004. Archived from the original on August 7, 2004. Retrieved March 2, 2013.
- ^ Leal, Walter S. (2005). "Pheromone Reception". In Schulz, Stefan (ed.). The Chemistry of Pheromones and Other Semiochemicals II. Topics in Current Chemistry. Vol. 240. Springer. pp. 1–36. doi:10.1007/b98314. ISBN 9783540213086. Retrieved March 2, 2013.
- ^ Caspi et al, Nucleic Acids Research 42:D459-D471 2014.
- ^ Ando, T; Hase, T; Arima, R; Uchiyama, M (1988). "Biosynthetic pathway of bombykol, the sex pheromone of the female silkworm moth". Agricultural and Biological Chemistry. 52 (2): 473–478. doi:10.1271/bbb1961.52.473.
- ^ Moto, K; Suzuki, MG; Hull, JJ; Kurata, R; Takahashi, S; Yamamoto, M; Okano, K; Imai, K; Ando, T; Matsumoto, S (2004). "Involvement of a bifunctional fatty-acyl desaturase in the biosynthesis of the silkmoth, Bombyx mori, sex pheromone". Proceedings of the National Academy of Sciences. 101 (23): 8631–6. Bibcode:2004PNAS..101.8631M. doi:10.1073/pnas.0402056101. PMC 423246. PMID 15173596.
- ^ a b Zhang, SD; Li, X; Bin, Z; Du, MF; Yin, XM; An, SH (2014). "Molecular identification of a pancreatic lipase-like gene involved in sex pheromone biosynthesis of Bombyx mori". Insect Science. 21 (4): 459–68. doi:10.1111/1744-7917.12053. PMID 23955937. S2CID 23364842.
- ^ a b Ohnishi, A; Kaji, M; Hashimoto, K; Matsumoto, S (2011). "Screening for the Genes Involved in Bombykol Biosynthesis: Identification and Functional Characterization of Bombyx mori Acyl Carrier Protein". Frontiers in Endocrinology. 2: 92. doi:10.3389/fendo.2011.00092. PMC 3355880. PMID 22649392.
- ^ Matsumoto, S; Ozawa, R; Uchiumi, K; Kurihara, M (1996). "Cell-free production of the silkworm sex pheromone bombykol". Bioscience, Biotechnology, and Biochemistry. 60 (2): 369–73. doi:10.1271/bbb.60.369. PMID 9063992.
