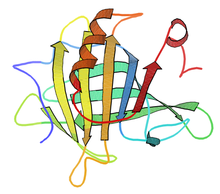
β-Lactoglobulin (BLG) is the major whey protein of cow and sheep's milk (~3 g/L), and is also present in many other mammalian species; a notable exception being humans. Its structure, properties and biological role have been reviewed many times.[1][2][3][4] BLG is considered to be a milk allergen.[5][6]
YouTube Encyclopedic
-
1/5Views:6 4991 1124531 061 534441
-
C3-03 milk proteins| Dairy technology
-
α Lactalbumin | Alpha Lactalbumin |
-
What does lactoglobulin mean?
-
The Worst Protein Powder for the Liver – Dr. Berg
-
Beta Lactoglobulina
Transcription
Function
The major protein in whey is β-lactoglobulin, followed by α-lactalbumin (β-lactoglobulin ≈ 65%, α-lactalbumin ≈ 25%, serum albumin ≈ 8%, other ≈ 2%). β-lactoglobulin is a lipocalin protein, and can bind many hydrophobic molecules, suggesting a role in their transport. β-lactoglobulin has also been shown to be able to bind iron via siderophores[7] and thus might have a role in combating pathogens. Upon ingestion BLG is able to shuttle complexed iron into human immune cells, thereby providing micronutrition to these cells and participating in immune tolerance.[8][9] A homologue of β-lactoglobulin is lacking in human breast milk.[10]
Structure
Several variants have been identified, the main ones in the cow being labelled A and B. Because of its abundance and ease of purification, it has been subjected to a wide range of biophysical studies. Its structure has been determined several times by X-ray crystallography and NMR.[11] β-lactoglobulin is of direct interest to the food industry since its properties can variously be advantageous or disadvantageous in dairy products and processing.[12]
Bovine β-lactoglobulin is a relatively small protein of 162 residues, with an 18.4 kDa. In physiological conditions it is predominantly dimeric, but dissociates to a monomer below about pH 3, preserving its native state as determined by using NMR.[13] Conversely, β-lactoglobulin also occurs in tetrameric,[14] octameric[15] and other multimeric[16] aggregation forms under a variety of natural conditions.
β-Lactoglobulin solutions form gels in various conditions, when the native structure is sufficiently destabilised to allow aggregation.[17] Under prolonged heating at low pH and low ionic strength, a transparent `fine-stranded' gel is formed, in which the protein molecules assemble into long stiff fibres.
β-Lactoglobulin is the main component of milk skin, coagulating and denaturing when the milk boils. Once denatured, the β-Lactoglobulin forms a thin gelatinous film on the surface of the milk.
Folding intermediates for this protein can be studied using light spectroscopy and denaturant. Such experiments show an unusual but important intermediate composed purely of alpha helices, despite the fact that the native structure is beta sheet. Evolution has probably selected for the helical intermediate to avoid aggregation during the folding process.[18]
Clinical significance
As milk is a known allergen,[5] manufacturers in European Union need to prove the presence or absence of β-lactoglobulin to ensure their labelling satisfies the requirements of the EC Directive. Food testing laboratories can use enzyme linked immunosorbent assay methods to identify and quantify β-lactoglobulin in food products. Though β-lactoglobulin is considered a major allergen, the protective impact of the consumption of raw milk has been shown to dependent on the protein-content of the whey fraction and thus of β-lactoglobulin.[19] This great contrast, on the one hand an allergen and on the other protective, has now been linked with its ability to carry micronutrient. When β-lactoglobulin carried micronutrient it acted tolerogenic and protected against allergy development. However, when the loading was missing, it turned into an allergen.[20][21][22]
Laboratory polymerization of β-lactoglobulin by microbial transglutaminase reduces its allergenicity in children and adults with an IgE-mediated cow’s milk allergy.[23]
Cows breeding
In 2018 it was announced that genetically modified cows were grown.[6] The cows had their β-Lactoglobulin producing genes removed by a zygote-mediated deletion process.[6]
See also
References
- ^ Xiang L, Melton L, Leung KH (2019). "Interactions of β-Lactoglobulin With Small Molecules". In Varelis P, Melton L, Shahidi F (eds.). Encyclopedia of Food Chemistry. Vol. 2. Elsevier. pp. 560–565. doi:10.1016/B978-0-08-100596-5.21488-1. ISBN 9780128140451. S2CID 90712856.
- ^ Sawyer L (1992). "Beta-lactoglobulin". In Fox PF, McSweeney PL (eds.). Advanced Dairy Chemistry: 1. Proteins. Elsevier Applied Science. pp. 141–190. doi:10.1007/978-1-4419-8602-3_7. ISBN 978-1-4419-8602-3.
- ^ Sawyer L, Kontopidis G (October 2000). "The core lipocalin, bovine beta-lactoglobulin". Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology. 1482 (1–2): 136–48. doi:10.1016/s0167-4838(00)00160-6. PMID 11058756.
- ^ Kontopidis G, Holt C, Sawyer L (April 2004). "Invited review: beta-lactoglobulin: binding properties, structure, and function". Journal of Dairy Science. 87 (4): 785–96. doi:10.3168/jds.S0022-0302(04)73222-1. PMID 15259212.
- ^ a b listed in Annex IIIa of Directive 2000/13/EC
- ^ a b c Wei, Jingwei; Wagner, Stefan; Maclean, Paul; Brophy, Brigid; Cole, Sally; Smolenski, Grant; Carlson, Dan F.; Fahrenkrug, Scott C.; Wells, David N.; Laible, Götz (2018-05-16). "Cattle with a precise, zygote-mediated deletion safely eliminate the major milk allergen beta-lactoglobulin". Scientific Reports. 8 (1): 7661. Bibcode:2018NatSR...8.7661W. doi:10.1038/s41598-018-25654-8. ISSN 2045-2322. PMC 5955954. PMID 29769555.
- ^ Roth-Walter F, Pacios LF, Gomez-Casado C, Hofstetter G, Roth GA, Singer J, et al. (2014-01-01). "The major cow milk allergen Bos d 5 manipulates T-helper cells depending on its load with siderophore-bound iron". PLOS ONE. 9 (8): e104803. Bibcode:2014PLoSO...9j4803R. doi:10.1371/journal.pone.0104803. PMC 4130594. PMID 25117976.
- ^ Roth-Walter F, Afify SM, Pacios LF, Blokhuis BR, Redegeld F, Regner A, et al. (January 2021). "Cow's milk protein β-lactoglobulin confers resilience against allergy by targeting complexed iron into immune cells". The Journal of Allergy and Clinical Immunology. 147 (1): 321–334.e4. doi:10.1016/j.jaci.2020.05.023. hdl:10261/272213. PMID 32485264. S2CID 219285403.
- ^ Afify SM, Pali-Schöll I, Hufnagl K, Hofstetter G, El-Bassuoni MA, Roth-Walter F, Jensen-Jarolim E (2021). "Bovine Holo-Beta-Lactoglobulin Cross-Protects Against Pollen Allergies in an Innate Manner in BALB/c Mice: Potential Model for the Farm Effect". Frontiers in Immunology. 12: 611474. doi:10.3389/fimmu.2021.611474. PMC 7977286. PMID 33746954.
- ^ Fiocchi A, Brozek J, Schünemann H, Bahna SL, von Berg A, Beyer K, et al. (April 2010). "World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow's Milk Allergy (DRACMA) Guidelines". The World Allergy Organization Journal. 3 (4): 57–161. doi:10.1097/WOX.0b013e3181defeb9. PMC 3488907. PMID 23268426.
- ^ PDB: 3BLG; Qin BY, Bewley MC, Creamer LK, Baker HM, Baker EN, Jameson GB (October 1998). "Structural basis of the Tanford transition of bovine beta-lactoglobulin". Biochemistry. 37 (40): 14014–23. doi:10.1021/bi981016t. PMID 9760236.
- ^ Jost R (1993). "Functional characteristics of dairy proteins". Trends in Food Science & Technology. 4 (9): 283–288. doi:10.1016/0924-2244(93)90071-H.
- ^ Uhrínová S, Smith MH, Jameson GB, Uhrín D, Sawyer L, Barlow PN (April 2000). "Structural changes accompanying pH-induced dissociation of the beta-lactoglobulin dimer". Biochemistry. 39 (13): 3565–74. doi:10.1021/bi992629o. PMID 10736155.
- ^ Timasheff SN, Townend R (1964). "Structure of the β-Lactoglobulin Tetramer". Nature. 203 (4944): 517–519. Bibcode:1964Natur.203..517T. doi:10.1038/203517a0. S2CID 4190604.
- ^ Gottschalk M, Nilsson H, Roos H, Halle B (November 2003). "Protein self-association in solution: the bovine beta -lactoglobulin dimer and octamer". Protein Science. 12 (11): 2404–11. doi:10.1110/ps.0305903. PMC 2366967. PMID 14573854.
- ^ Rizzuti B, Zappone B, De Santo MP, Guzzi R (January 2010). "Native beta-lactoglobulin self-assembles into a hexagonal columnar phase on a solid surface". Langmuir. 26 (2): 1090–5. doi:10.1021/la902464f. PMID 19877696.
- ^ Bromley EH, Krebs MR, Donald AM (2005). "Aggregation across the length-scales in beta-lactoglobulin". Faraday Discussions. 128: 13–27. doi:10.1039/b403014a. PMID 15658764.
- ^ Kuwajima K, Yamaya H, Sugai S (December 1996). "The burst-phase intermediate in the refolding of beta-lactoglobulin studied by stopped-flow circular dichroism and absorption spectroscopy". Journal of Molecular Biology. 264 (4): 806–22. doi:10.1006/jmbi.1996.0678. PMID 8980687.
- ^ Loss G, Apprich S, Waser M, Kneifel W, Genuneit J, Büchele G, et al. (October 2011). "The protective effect of farm milk consumption on childhood asthma and atopy: the GABRIELA study". The Journal of Allergy and Clinical Immunology. 128 (4): 766–773.e4. doi:10.1016/j.jaci.2011.07.048. hdl:1874/407013. PMID 21875744.
- ^ Roth-Walter F, Afify SM, Pacios LF, Blokhuis BR, Redegeld F, Regner A, et al. (January 2021). "Cow's milk protein β-lactoglobulin confers resilience against allergy by targeting complexed iron into immune cells". The Journal of Allergy and Clinical Immunology. 147 (1): 321–334.e4. doi:10.1016/j.jaci.2020.05.023. hdl:10261/272213. PMID 32485264.
- ^ Hufnagl K, Ghosh D, Wagner S, Fiocchi A, Dahdah L, Bianchini R, et al. (January 2018). "Retinoic acid prevents immunogenicity of milk lipocalin Bos d 5 through binding to its immunodominant T-cell epitope". Scientific Reports. 8 (1): 1598. Bibcode:2018NatSR...8.1598H. doi:10.1038/s41598-018-19883-0. PMC 5785490. PMID 29371615.
- ^ Afify SM, Pali-Schöll I, Hufnagl K, Hofstetter G, El-Bassuoni MA, Roth-Walter F, Jensen-Jarolim E (2021). "Bovine Holo-Beta-Lactoglobulin Cross-Protects Against Pollen Allergies in an Innate Manner in BALB/c Mice: Potential Model for the Farm Effect". Frontiers in Immunology. 12: 611474. doi:10.3389/fimmu.2021.611474. PMC 7977286. PMID 33746954.
- ^ Olivier CE, Lima RP, Pinto DG, Santos RA, Silva GK, Lorena SL, et al. (October 2012). "In search of a tolerance-induction strategy for cow's milk allergies: significant reduction of beta-lactoglobulin allergenicity via transglutaminase/cysteine polymerization". Clinics. 67 (10): 1171–9. doi:10.6061/clinics/2012(10)09. PMC 3460020. PMID 23070344.