| |
| Names | |
|---|---|
| Preferred IUPAC name
Hexadehydrobenzene | |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6 | |
| Molar mass | 72.066 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
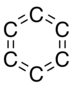
Benzotriyne or cyclo[6]carbon is a hypothetical compound, an allotrope of carbon with molecular formula C6. The molecule is a ring of six carbon atoms, connected by alternating triple and single bonds.[1] It is, therefore, a potential member of the cyclo[n]carbon family.
There have been a few attempts to synthesize benzotriyne, e.g. by pyrolysis of mellitic anhydride,[2] but without success as of 2011[update]. Calculations suggest that the alternative cyclic cumulene structure, called cyclohexahexaene, is the potential energy minimum of the cyclo[6]carbon framework. It is unclear whether this second structure is an isomer or a limiting resonance structure.[3]
References
- ^ Adamson, George A.; Rees, Charles W. (1996). "Towards the total synthesis of cyclo[n]carbons and the generation of cyclo[6]carbon". J. Chem. Soc., Perkin Trans. 1 (13): 1535–1543. doi:10.1039/P19960001535.
- ^ Fields, Ellis K.; Meyerson, Seymour (October 1966). "Arynes by Pyrolysis of Acid Anhydrides". J. Org. Chem. 31 (10): 3307–3309. doi:10.1021/jo01348a046.
- ^ Zahradník, Rudolf; Hobza, Pavel; Burcl, Rudolf; Andes Hess, B. (October 1994). "Strained unsaturated molecules. Theoretical study of acyclic and cyclic cumulenes and acetylenes". Journal of Molecular Structure: THEOCHEM. 313 (3): 335–349. doi:10.1016/0166-1280(94)85015-1.