 | |
| Clinical data | |
|---|---|
| Trade names | Balversa |
| Other names | JNJ-42756493 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a619031 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Organonitrogen compounds |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| PubChem SID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| ECHA InfoCard | 100.235.008 |
| Chemical and physical data | |
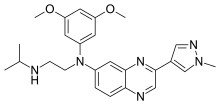
| Formula | C25H30N6O2 |
| Molar mass | 446.555 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Erdafitinib, sold under the brand name Balversa, is an anti-cancer medication. It is a small molecule inhibitor of fibroblast growth factor receptor (FGFR) used for the treatment of cancer. FGFRs are a subset of tyrosine kinases which are unregulated in some tumors and influence tumor cell differentiation, proliferation, angiogenesis, and cell survival.[3][4] Astex Pharmaceuticals discovered the drug[5] and licensed it to Janssen Pharmaceuticals for further development.[3]
Researchers have investigated erdafitinib for safety and efficacy in treatment of bile duct cancer, gastric cancer, non-small cell lung cancer, and esophageal cancer.[6]
YouTube Encyclopedic
-
1/5Views:1 349403413385564
-
Erdafitinib for FGFR-mutated urothelial carcinoma
-
An update on erdafitinib for metastatic/uresectable FGFR alteration urothelial carcinoma
-
Dr. Sonpavde on the Safety Profile of Erdafitinib in Bladder Cancer
-
Erdafitinib for bladder cancer: more to come following FDA approval
-
Dr. Siefker-Radtke on the Phase II Study of Erdafitinib in Patients With Urothelial Carcinoma
Transcription
Medical uses
In April 2019, erdafitinib was granted approval by the US Food and Drug Administration (FDA) for treatment of metastatic or locally advanced bladder cancer with an FGFR3 or FGFR2 alteration that has progressed beyond traditional platinum-based therapies, subject to a confirmatory trial.[4][7][8] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[9]
In January 2024, the FDA approved erdafitinib for adults with locally advanced or metastatic urothelial carcinoma with susceptible FGFR3 genetic alterations, as determined by an FDA-approved companion diagnostic test, whose disease has progressed on or after at least one line of prior systemic therapy.[10]
Side effects
Common side effects include increased phosphate level, mouth sores, feeling tired, change in kidney function, diarrhea, dry mouth, nails separating from the bed or poor formation of the nail, change in liver function, low salt (sodium) levels, decreased appetite, change in sense of taste, low red blood cells (anemia), dry skin, dry eyes and hair loss.[4] Other side effects include redness, swelling, peeling or tenderness on the hands or feet (hand foot syndrome), constipation, stomach pain, nausea and muscle pain.[4]
Erdafitinib may cause serious eye problems, including inflamed eyes, inflamed cornea (front part of the eye) and disorders of the retina, an internal part of the eye.[4] Patients are advised to have eye examinations intermittently and to tell their health care professional right away if they develop blurred vision, loss of vision or other visual changes.[4]
History
The efficacy of erdafitinib was studied in a clinical trial (NCT02365597) that included 87 adults with locally advanced or metastatic bladder cancer, with FGFR3 or FGFR2 genetic alterations, that had progressed following treatment with chemotherapy.[4][11] The overall response rate in these adults was 32.2%, with 2.3% having a complete response and almost 30% having a partial response.[4] The response lasted for a median of approximately five-and-a-half months.[4] The trial was conducted in Asia, Europe, and the United States.[11]
Erdafitinib received an accelerated approval.[4] Further clinical trials are required to confirm erdafitinib's clinical benefit and the sponsor is conducting or plans to conduct these studies.[4] Erdafitinib was also granted breakthrough therapy designation.[4] The FDA granted the approval of Balversa to Janssen Pharmaceutical.[4][8] The FDA also approved the therascreen FGFR RGQ RT-PCR Kit, developed by Qiagen Manchester, Ltd., for use as a companion diagnostic with erdafinitib for this therapeutic indication.[4]
In January 2024, the FDA approved erdafitinib for adults with locally advanced or metastatic urothelial carcinoma with susceptible FGFR3 genetic alterations, as determined by an FDA-approved companion diagnostic test, whose disease has progressed on or after at least one line of prior systemic therapy.[10] This approval amends the indication previously granted under accelerated approval for people with metastatic urothelial carcinoma with susceptible FGFR3 or FGFR2 alterations after prior platinum-containing chemotherapy.[10] Efficacy was evaluated in Study BLC3001 Cohort 1, a randomized, open-label trial of 266 participants with metastatic urothelial carcinoma harboring selected FGFR3 alterations who had received 1-2 prior systemic treatments, including a PD-1 or PD-L1 inhibitor.[10] Participants were randomized 1:1 to receive erdafitinib or investigator's choice of chemotherapy (docetaxel or vinflunine).[10] Randomization was stratified by region, performance status, and presence of visceral or bone metastases.[10] FGFR3 alterations were identified from tumor tissue in a central laboratory by the therascreen FGFR RGQ RT-PCR kit (Qiagen) in 75% of participants, while the remainder were identified by local next generation sequencing assays.[10]
Society and culture
Legal status
In March 2018, erdafitinib was granted breakthrough therapy designation by the FDA for treatment of urothelial cancer.[3]
References
- ^ "Balversa Product information". Health Canada. Retrieved 29 May 2022.
- ^ "Summary Basis of Decision (SBD) for Balversa". Health Canada. 23 October 2014. Retrieved 29 May 2022.
- ^ a b c "Janssen Announces U.S. FDA Breakthrough Therapy Designation for Erdafitinib in the Treatment of Metastatic Urothelial Cancer". Johnson & Johnson (Press release). Archived from the original on 20 June 2018.
- ^ a b c d e f g h i j k l m n "FDA approves first targeted therapy for metastatic bladder cancer". U.S. Food and Drug Administration (FDA) (Press release). 12 April 2019. Archived from the original on 15 November 2019. Retrieved 13 May 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Saxty G (3 November 2011). "Pyrazolyl quinazoline kinase inhibitors". Google Patents.
- ^ Bahleda R, Italiano A, Hierro C, Mita A, Cervantes A, Chan N, et al. (August 2019). "Multicenter Phase I Study of Erdafitinib (JNJ-42756493), Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients with Advanced or Refractory Solid Tumors". Clin. Cancer Res. 25 (16): 4888–97. doi:10.1158/1078-0432.CCR-18-3334. hdl:10854/7722. PMID 31088831. S2CID 155089088.
- ^ "Balversa (erdafitinib) Receives U.S. FDA Approval for the Treatment of Patients with Locally Advanced or Metastatic Urothelial Carcinoma with Certain FGFR Genetic Alterations". Johnson & Johnson (Press release). 8 May 2019. Archived from the original on 8 May 2019. Retrieved 24 November 2019.
- ^ a b "Drug Approval Package: Balversa (erdafinitib)". U.S. Food and Drug Administration (FDA).
- ^ "New Drug Therapy Approvals 2019". U.S. Food and Drug Administration (FDA). 31 December 2019. Retrieved 15 September 2020.
- ^ a b c d e f g "FDA approves erdafitinib for urothelial carcinoma". U.S. Food and Drug Administration. 19 January 2024. Retrieved 9 March 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b "Drug Trials Snapshots: Balversa". U.S. Food and Drug Administration (FDA). 12 April 2019. Archived from the original on 27 September 2019. Retrieved 24 November 2019.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
