| |
| |
| Names | |
|---|---|
| IUPAC name
2,2′-Azobis(2-methylpropionitrile), 2-(azo(1-cyano-1-methylethyl))-2-methylpropane nitrile
| |
| Other names
Azobisisobutyronitrile
Azobisisobutylonitrile AIBN | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | AIBN |
| ChemSpider | |
| ECHA InfoCard | 100.001.030 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 3234 1325 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H12N4 | |
| Molar mass | 164.21 g/mol |
| Appearance | white crystals |
| Density | 1.1 g cm−3 |
| Melting point | 103 to 105 °C (217 to 221 °F; 376 to 378 K) |
| poor | |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| H242, H302, H332, H412 | |
| P210, P220, P234, P261, P264, P270, P271, P273, P280, P301+P312, P304+P312, P304+P340, P312, P330, P370+P378, P403+P235, P411, P420, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Azobisisobutyronitrile (abbreviated AIBN) is an organic compound with the formula [(CH3)2C(CN)]2N2. This white powder is soluble in alcohols and common organic solvents but is insoluble in water. It is often used as a foamer in plastics and rubber and as a radical initiator.
As an azo initiator, radicals resulting from AIBN have multiple benefits[1] over common organic peroxides. For example, they do not have oxygenated byproducts or much yellow discoloration. Additionally, they do not cause too much grafting and therefore are often used when making adhesives, acrylic fibers, detergents, etc.
YouTube Encyclopedic
-
1/3Views:9567 90921 347
-
Azobisisobutyronitrile
-
Tributyl tin hydride Bu3SnH, Bu3SnH AIBN Reactions, Mechanism, Chemoselectivity
-
Recrystallization Demonstrated by Mark Niemczyk, PhD
Transcription
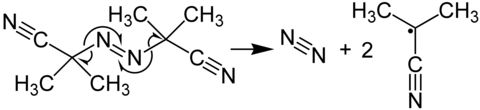
Mechanism of decomposition
In its most characteristic reaction, AIBN decomposes, eliminating a molecule of nitrogen gas to form two 2-cyanoprop-2-yl radicals:
Because azobisisobutyronitrile readily gives off free radicals, it is often used as a radical initiator. This happens at temperatures above 40 °C,[2] but in experiments is more commonly done at temperatures between 66 °C and 72 °C.[3] This decomposition has a ΔG‡ of 131 kJ/mol[3] and results in two 2-cyano-2-propyl (carbon) radicals and a molecule of nitrogen gas. The release of nitrogen gas pushes this decomposition forward due to the increase in entropy. And the 2-cyano-2-propyl radical is stabilized by the −CN group.
Chemical reactions
These radicals formed by the decomposition of AIBN can initiate free-radical polymerizations and other radical-induced reactions. For instance, a mixture of styrene and maleic anhydride in toluene will react if heated, forming the copolymer upon addition of AIBN. Another example of a radical reaction that can be initiated by AIBN is the anti-Markovnikov hydrohalogenation of alkenes. AIBN has also been used as the radical initiator for Wohl–Ziegler bromination. The AIBN-derived 2-cyano-2-propyl radical abstracts the hydrogen from tributyltin hydride.[4] The resulting tributyltin radical can be used for removal of a bromine atom.
AIBN-derived radicals abstract a hydrogen from HBr to give a bromine radical, which can add to alkenes. This type of hydrohalogenation of an alkene proceeds with anti-Markovnikov selectivity.
Production and analogues
AIBN is produced in two steps from acetone cyanohydrin. Reaction with hydrazine gives the substituted dialkylhydrazine. In the second step, the hydrazine is oxidized to the azo derivative:[5][6]
- 2 (CH3)2C(CN)OH + N2H4 → [(CH3)2C(CN)]2N2H2 + 2 H2O
- [(CH3)2C(CN)]2N2H2 + Cl2 → [(CH3)2C(CN)]2N2 + 2 HCl
Related azo compounds behave similarly, such as 1,1′-azobis(cyclohexanecarbonitrile). Water-soluble azo initiators are also available.[7][8]
Safety
AIBN is safer to use than benzoyl peroxide (another radical initiator) because the risk of explosion is far less. However, it is still considered as an explosive compound, decomposing above 65 °C. A respirator dust mask, protective gloves and safety glasses are recommended. Pyrolysis of AIBN without a trap for the formed 2-cyanopropyl radicals results in the formation of tetramethylsuccinonitrile, which is highly toxic.
References
- ^ AIBN initiator and other azo initiators. (n.d.). Retrieved from https://polymerchemistry.nouryon.com/products-applications/acrylic-polymer-initiators/aibn/
- ^ 2,2′-Azobis(2-methylpropionitrile) 441090. (n.d.). Retrieved from https://www.sigmaaldrich.com/catalog/product/aldrich/441090?lang=en®ion=US
- ^ a b Clayden, J., Greeves, N., & Warren, S. (2017). Organic chemistry. MTM.
- ^ Giese, Bernd; Gröninger, Kay S. (1990). "1,3,4,6-TETRA-O-ACETYL-2-DEOXY-a-D-GLUCOPYRANOSE". Organic Syntheses. 69: 66. doi:10.15227/orgsyn.069.0066.
- ^ Schirmann, Jean-Pierre; Bourdauducq, Paul. "Hydrazine". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_177. ISBN 978-3527306732.
- ^ Overberger, C. G., O'Shaughnessy, M. T., Shalit, H. (1949). The Preparation of Some Aliphatic Azo Nitriles and their Decomposition in Solution. Journal of the American Chemical Society, 71(8), 2661-2666. doi:10.1021/ja01176a018
- ^ "Vazo Product Grades". www2.dupont.com. Archived from the original on 2009-03-26. Retrieved 2007-12-15.
- ^ Water-soluble Azo initiators
External links
- SIDS Initial Assessment Report for 2,2′-Azobis(2-methylpropionitrile) from the Organisation for Economic Co-operation and Development (OECD)