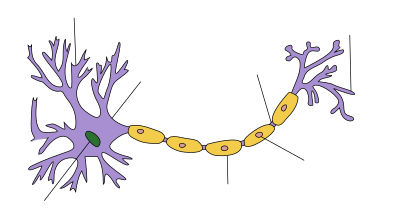
Axon terminals (also called synaptic boutons, presynaptic terminals, or end-feet) are distal terminations of the branches of an axon. An axon, also called a nerve fiber, is a long, slender projection of a nerve cell that conducts electrical impulses called action potentials away from the neuron's cell body in order to transmit those impulses to other neurons, muscle cells or glands. In the central nervous system, most presynaptic terminals are actually formed along the axons (en-passant boutons), not at their ends (terminal boutons).
Functionally, the axon terminal converts an electrical signal into a chemical signal. When an action potential arrives at an axon terminal (A), neurotransmitter is released and diffuses across the synaptic cleft. If the postsynaptic cell (B) is also a neuron, neurotransmitter receptors generate a small electrical current that changes the postsynaptic potential. If the postsynaptic cell (B) is a muscle cell (neuromuscular junction), it contracts.
YouTube Encyclopedic
-
1/5Views:3 660 670165 673131 28366 283112 468
-
The Nervous System, Part 3 - Synapses!: Crash Course A&P #10
-
Neural Conduction, Action Potential, and Synaptic Transmission
-
2-Minute Neuroscience: Neurotransmitter Release
-
Synaptic Terminal (Neuromuscular Junction)
-
Somatic Nervous System
Transcription
What’s 1000 times thinner than a piece of paper, more numerous in you than grains of sand on a beach, and proof that the smallest things can sometimes be the most powerful? I’m talking about the synapse -- the meeting point between two neurons. If your neurons form the structure of your nervous system, then your synapses -- the tiny communication links between them -- are what turn that structure into an actual system. Because, as great and powerful as your neurons are, when it comes down to it, their strength and their purpose lies in their connections. A single neuron in isolation might as well not exist if it doesn’t have someone to listen or talk to. The word “synapse” comes from the Greek for “to clasp or join.” It’s basically a junction or a crossroads. When an action potential -- and if you don’t know what an action potential is, watch the last episode -- sends an electrical message to the end of an axon, that message hits a synapse that then translates, or converts it, into a different type of signal and flings it over to another neuron. These connections are rather amazing feats of bio-electrical engineering, and they are also ridiculously, mind-bogglingly numerous. Consider that the human brain alone has 100 billion neurons, and each of those has 1000 to 10,000 synapses. So you’ve got somewhere between 100 to 1,000 trillion synapses in your brain. Each one of these hundreds of trillions of synapses is like a tiny computer, all of its own, not only capable of running loads of different programs simultaneously, but also able to change and adapt in response to neuron firing patterns, and either strengthen or weaken over time, depending on how much they’re used. Synapses are what allow you to learn and remember. They’re also the root of many psychiatric disorders. And they’re basically why illicit drugs -- and addictions to them -- exist. Pretty much everything in your experience -- from euphoria to hunger to desire to fuzziness to to confusion to boredom -- is communicated by way of these signals sent by your body’s own electrochemical messaging system. Hopefully, you know enough about email and texting etiquette to know that if you’re gonna communicate effectively, you have to respect the sanctity of the group list. It’s not a great idea to send a mass text to all of your friends first thing in the morning to give them the urgent news that you just ate a really delicious maple-bacon donut. Seriously, people. If you happen to have a friend who truly adores bacon, then an email would suffice. But! If you’re out clubbing and suddenly Bill Murray shows up and starts doing karaoke... then that would be a totally appropriate time to notify all of your friends at once that something awesome is happening and they better be a part of it. And in much the same way -- OK, in kind of the same way -- your nerve cells have two main settings for communicating with each other, depending on how fast the news needs to travel. Some of your synapses are electrical -- that would be like an immediate group text. Others are chemical synapses -- they take more time to be received and read, but they’re used more often and are much easier to control, sending signals to only certain recipients. Fortunately, your nervous system has better text etiquette than your mom, and knows when each kind is appropriate to use, and how to do it. Your super fast electrical synapses send an ion current flowing directly from the cytoplasm of one nerve cell to another, through small windows called gap junctions. They’re super fast because the signal is never converted from its pure electrical state to any other kind of signal, the way it is in a chemical synapse. Instead, one cell and one synapse can trigger thousands of other cells that can all act in synchrony. Something similar happens in the muscle cells of your heart, where speed and team effort between cells is crucial. This seems like a good system, so why aren’t all of our synapses electrical? It’s largely a matter of control. With such a direct connection between cells, an action potential in one neuron will generate an action potential in the other cells across the synapse. That’s great in places like your heart, because you definitely don’t want a half a heartbeat. But if every synapse in your body activated all of the neurons around it, your nervous system would basically always be in “group text” mode, with every muscle fiber and bit of organ tissue always being stimulated and then replying-all to the whole group which would stimulate them even more until everyone just got maxed out and exhausted and turned off their phones for good...which would be death. So that would be bad, which is partly why we have chemical synapses. They are much more abundant, but also slower, and they’re more precise and selective in what messages they send where. Rather than raw electricity, these synapses use neurotransmitters, or chemical signals, that diffuse across a synaptic gap to deliver their message. The main advantage chemical synapses have over electrical ones is that they can effectively convert the signal in steps -- from electrical to chemical back to electrical -- which allows for different ways to control that impulse. At the synapse, that signal can be modified, amplified, inhibited, or split, either immediately or over longer periods of time. This set-up has two principal parts: The cell that’s sending the signal is the presynaptic neuron, and it transmits through a knoblike structure called the presynaptic terminal, usually the axon terminal. This terminal holds a whole bunch of tiny synaptic vesicle sacs, each loaded with thousands of molecules of a given neurotransmitter. The receiving cell, meanwhile, is, yes, thankfully the postsynaptic neuron, and it accepts the neurotransmitters in its receptor region, which is usually on the dendrite or just on the cell body itself. And these two neurons communicate even though they never actually touch. Instead, there’s a tiny gap called a synaptic cleft between them -- less than five millionths of a centimeter apart. One thing to remember is that messages that travel via chemical synapses are technically not transmitted directly between neurons, like they are in electrical synapses. Instead, there’s a whole chemical event that involves the release, diffusion, and reception of neurotransmitters in order to transmit signals. And this all happens in a specific and important chain of events. When an action potential races along the axon of a neuron, activating sodium and potassium channels in a wave, it eventually comes down to the presynaptic terminal, and activates the voltage-gated calcium (Ca2+) channels there to open and release the calcium into the neuron’s cytoplasm. This flow of positively-charged calcium ions causes all those tiny synaptic vesicles to fuse with the cell membrane and purge their chemical messengers. And it’s these neurotransmitters that act like couriers diffusing across the synaptic gap, and binding to receptor sites on the postsynaptic neuron. So, the first neuron has managed to convert the electrical signal into a chemical one. But in order for it to become an action potential again in the receiving neuron, it has to be converted back to electrical. And that happens once a neurotransmitter binds to a receptor. Because, that’s what causes the ion channels to open. And depending on which particular neurotransmitter binds to which receptor, the neuron might either get excited or inhibited. The neurotransmitter tells it what to do. Excitatory neurotransmitters depolarize the postsynaptic neuron by making the inside of it more positive and bringing it closer to its action potential threshold, making it more likely to fire that message on to the next neuron. But an inhibitory neurotransmitter hyperpolarizes the postsynaptic neuron by making the inside more negative, driving its charge down -- away from its threshold. So, not only does the message not get passed along, it’s now even harder to excite that portion of the neuron. Keep in mind here: Any region of a single neuron may have hundreds of synapses, each with different inhibitory or excitatory neurotransmitters. So the likelihood of that post-synaptic neuron developing an action potential depends on the sum of all of the excitations and inhibitions in that area. Now, we have over a hundred different kinds of naturally-occurring neurotransmitters in our bodies that serve different functions. They help us move around, and keep our vital organs humming along, amp us up, calm us down, make us hungry, sleepy, or more alert, or simply just make us feel good. But neurotransmitters don’t stay bonded to their receptors for more than a few milliseconds. After they deliver their message, they just sort of pop back out, and then either degrade or get recycled. Some kinds diffuse back across the synapse and are immediately re-absorbed by the sending neuron, in a process called reuptake. Others are broken down by enzymes in the synaptic cleft, or sent away from the synapse by diffusion. And this mechanism is what many drugs -- both legal and illegal -- so successfully exploit, in order to create their desired effects. These drugs can either excite or inhibit the production, release, and reuptake of neurotransmitters. And sometimes they can simply mimic neurotransmitters, tricking a neuron into thinking it’s getting a natural chemical signal, when really it’s anything but. Take cocaine, for example. Don’t take cocaine. Once it hits your bloodstream, it targets three major neurotransmitters -- serotonin, dopamine, and norepinephrine. Serotonin is mainly inhibitory and plays an important role in regulating mood, appetite, circadian rhythm, and sleep. Some antidepressants can help stabilize moods by stabilizing serotonin levels. And when you engage in pleasurable activities -- like hugging a loved one, or having sex, or eating a really, really great donut -- your brain releases dopamine, which influences emotion and attention, but mostly just makes you feel awesome. Finally, norepinephrine amps you up by triggering your fight or flight response, increasing your heart rate, and priming muscles to engage, while an undersupply of the chemical can depress a mood. So in a normal, sober state, you’ve got all these neurotransmitters doing their thing in your body. But once they’ve delivered their chemical payloads, they’re usually diffused right back out across the synapse to be absorbed by the neuron that sent them. But cocaine blocks that reuptake, especially of dopamine, allowing these powerful chemicals to float around and accumulate -- making the user feel euphoric for a time, but also paranoid and jittery. And because you have a limited supply of these neurotransmitters, and your body needs time to brew more, flooding your synapses like this eventually depletes your supply, making you feel terrible in a number of ways. Cocaine and other drugs that target neurotransmitters trick the brain, and after prolonged use may eventually cause it to adapt, as all those synapses remember how great those extra chemicals feel. As a result, you actually start to lose receptors, so it takes even more dopamine, and finally cocaine, to function normally. Sometimes the best way to understand how your body works is to look at how things can go wrong. And when it comes to your synapses, that, my friends, is what wrong looks like. In their natural, healthy state, your synapses know when to excite, when to inhibit, when to use electricity and when to dispatch the chemical messengers. Basically, a healthy nervous system has the etiquette of electrical messaging down to, well, a science. Today you learned how electrical synapses use ion currents over gap junctions to transmit neurological signals, and how chemical synapses turn electrical signals into chemical ones, using neurotransmitters, before converting them to back electrical signals again. And you learned how cocaine is a sterling example of how artificial imbalances in this electrochemical system can lead to dysfunctions of all kinds. This episode of Crash Course was brought to you by Logan Sanders from Branson, MO, and Dr. Linnea Boyev, whose YouTube channel you can check out in the description below. Thank you to Logan and Dr. Boyev for supporting Crash Course and free education. Thank you to everyone who's watching, but especially to our Subbable subscribers, like Logan and Dr. Boyev, who make Crash Course possible. To find out how you can become a supporter, just go to Subbable.com. This episode was written by Kathleen Yale, the script was edited by Blake de Pastino, and our consultant, is Dr. Brandon Jackson. It was directed by Nicholas Jenkins and Michael Aranda, and our graphics team is Thought Café.
Neurotransmitter release
Axon terminals are specialized to release neurotransmitter very rapidly by exocytosis.[1] Neurotransmitter molecules are packaged into synaptic vesicles that cluster beneath the axon terminal membrane on the presynaptic side (A) of a synapse. Some of these vesicles are docked, i.e. connected to the membrane by a number of specialized proteins, the SNARE complex. The incoming action potential activates voltage-gated calcium channels, leading to an influx of calcium ions into the axon terminal. The SNARE complex reacts to these calcium ions and forces the membrane of the vesicle to fuse with the presynaptic membrane, releasing their content into the synaptic cleft within 180 µs of calcium entry.[2][3][4] When receptors in the postsynaptic membrane bind this neurotransmitter and open ion channels, information has been transmitted between neuron (A) and neuron (B).[5] To generate an action potential in the postsynaptic neuron, many excitatory synapses must be active at the same time.[1]
Imaging the activity of axon terminals
Historically, calcium-sensitive dyes were the first tool to quantify the calcium influx into synaptic terminals and to investigate the mechanisms of short-term plasticity.[6] The process of exocytosis can be visualized with pH-sensitive fluorescent proteins (Synapto-pHluorin): Before release, vesicles are acidic and the fluorescence is quenched. Upon release, they are neutralized, generating a brief flash of green fluorescence.[7] Another possibility is to construct a genetically encoded sensor that becomes fluorescent when bound to a specific neurotransmitter, e.g. glutamate.[8] This method is sensitive enough to detect the fusion of a single transmitter vesicle in brain tissue and to measure the release probability at individual synapses.[9]
See also
- Calyx of Held, a giant axon terminal in the auditory system
- Neuromuscular junction, axon terminal contacting a muscle cell
- Endocytosis to recycle vesicles after use
- Vesicular monoamine transporter, loading vesicles with neurotransmitter
- Optogenetic methods to measure cellular activity
References
- ^ a b Purves, Dale; Augustine, George J.; Fitzpatrick, David, eds. (2019). Neuroscience (6th ed.). New York: Sinauer Associates / Oxford University Press. ISBN 978-1-60535-841-3.
- ^ Llinás R, Steinberg IZ, Walton K (March 1981). "Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse". Biophysical Journal. 33 (3): 323–351. Bibcode:1981BpJ....33..323L. doi:10.1016/S0006-3495(81)84899-0. PMC 1327434. PMID 6261850.
- ^ Rizo J (August 2018). "Mechanism of neurotransmitter release coming into focus". Protein Science (Review). 27 (8): 1364–1391. doi:10.1002/pro.3445. PMC 6153415. PMID 29893445.
Research for three decades and major recent advances have provided crucial insights into how neurotransmitters are released by Ca2+ -triggered synaptic vesicle exocytosis, leading to reconstitution of basic steps that underlie Ca2+ -dependent membrane fusion and yielding a model that assigns defined functions for central components of the release machinery.
- ^ Südhof TC, Rizo J (December 2011). "Synaptic vesicle exocytosis". Cold Spring Harbor Perspectives in Biology. 3 (12): a005637. doi:10.1101/cshperspect.a005637. PMC 3225952. PMID 22026965.
- ^ Siegelbaum, Steven A. (2021). Kandel, Eric R.; Koester, John D.; Mack, Sarah H. (eds.). Principles of neural science (6th ed.). New York: McGraw-Hill. ISBN 978-1-259-64223-4.
- ^ Zucker RS, Regehr WG (2002). "Short-term synaptic plasticity". Annual Review of Physiology. 64 (1): 355–405. doi:10.1146/annurev.physiol.64.092501.114547. PMID 11826273.
- ^ Burrone J, Li Z, Murthy VN (2006). "Studying vesicle cycling in presynaptic terminals using the genetically encoded probe synaptopHluorin". Nature Protocols. 1 (6): 2970–2978. doi:10.1038/nprot.2006.449. PMID 17406557. S2CID 29102814.
- ^ Marvin JS, Borghuis BG, Tian L, Cichon J, Harnett MT, Akerboom J, et al. (February 2013). "An optimized fluorescent probe for visualizing glutamate neurotransmission". Nature Methods. 10 (2): 162–170. doi:10.1038/nmeth.2333. PMC 4469972. PMID 23314171.
- ^ Dürst CD, Wiegert JS, Schulze C, Helassa N, Török K, Oertner TG (October 2022). "Vesicular release probability sets the strength of individual Schaffer collateral synapses". Nature Communications. 13 (1): 6126. doi:10.1038/s41467-022-33565-6. PMC 9576736. PMID 36253353.
Further reading
- Cragg SJ, Greenfield SA (August 1997). "Differential autoreceptor control of somatodendritic and axon terminal dopamine release in substantia nigra, ventral tegmental area, and striatum". The Journal of Neuroscience. 17 (15): 5738–5746. doi:10.1523/JNEUROSCI.17-15-05738.1997. PMC 6573186. PMID 9221772.
- Vaquero CF, de la Villa P (October 1999). "Localisation of the GABA(C) receptors at the axon terminal of the rod bipolar cells of the mouse retina". Neuroscience Research. 35 (1): 1–7. doi:10.1016/S0168-0102(99)00050-4. PMID 10555158. S2CID 53189471.
- Roffler-Tarlov S, Beart PM, O'Gorman S, Sidman RL (May 1979). "Neurochemical and morphological consequences of axon terminal degeneration in cerebellar deep nuclei of mice with inherited Purkinje cell degeneration". Brain Research. 168 (1): 75–95. doi:10.1016/0006-8993(79)90129-X. PMID 455087. S2CID 19618884.
- Yagi T, Kaneko A (February 1988). "The axon terminal of goldfish retinal horizontal cells: a low membrane conductance measured in solitary preparations and its implication to the signal conduction from the soma". Journal of Neurophysiology. 59 (2): 482–494. doi:10.1152/jn.1988.59.2.482. PMID 3351572.
- LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite.Toni N, Buchs PA, Nikonenko I, Bron CR, Muller D (November 1999). "LTP promotes formation of multiple spine synapses between a single axon terminal and a dendrite". Nature. 402 (6760): 421–425. Bibcode:1999Natur.402..421T. doi:10.1038/46574. PMID 10586883. S2CID 205056308.