 | |
 | |
| Combination of | |
|---|---|
| Amlodipine | Calcium channel blocker |
| Valsartan | Angiotensin II receptor antagonist |
| Clinical data | |
| Trade names | Exforge |
| AHFS/Drugs.com | Professional Drug Facts |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| (verify) | |
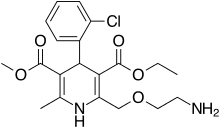
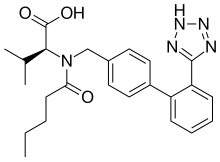
Amlodipine/valsartan, sold under the brand name Exforge among others, is a blood pressure lowering combination drug. It contains amlodipine, as the besilate, a dihydropyridine-type calcium channel blocker, and valsartan, an angiotensin II receptor antagonist.[3] This combination is usually well tolerated and effective for the reduction of blood pressure.[4]
The combination was approved for medical use in the United States in June 2007.[5]
The combination is also available with included hydrochlorothiazide (Exforge HCT), for people who need a three-drug regimen to manage their blood pressure.[6][7] It was approved for medical use in the United States in April 2009.[8]
YouTube Encyclopedic
-
1/3Views:53 36020 55645 458
-
How does Amlodipine work?
-
Amlodipine (norvasc) Side Effects
-
ARBS Angiotensin Receptor Blockers
Transcription
References
- ^ "Amlodipine / valsartan (Exforge) Use During Pregnancy". Drugs.com. 11 July 2019. Retrieved 13 February 2020.
- ^ "Exforge- amlodipine besylate and valsartan tablet, film coated". DailyMed. 12 June 2019. Retrieved 12 February 2020.
- ^ "Valsartan Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 3 March 2019.
- ^ Eckert S, Freytag SB, Müller A, Klebs SH (September 2013). "Meta-analysis of three observational studies of amlodipine/valsartan in hypertensive patients with additional risk factors". Blood Pressure. 22 (sup1): 11–21. doi:10.3109/08037051.2013.793891. PMID 23713686. S2CID 25814898.
- ^ "Drug Approval Package: Exforge (amlodipine and valsartan) NDA #021990". U.S. Food and Drug Administration (FDA). 3 October 2008. Retrieved 19 September 2021.
- ^ "Exforge HCT- amlodipine valsartan and hydrochlorothiazide tablet, film coated". DailyMed. Retrieved 12 February 2020.
- ^ "Amlodipine, Valsartan, and Hydrochlorothiazide". Drugs.com.
- ^ "Drug Approval Package: Exforge HCT (Amlodipine, Valsartan, Hydrochlorothiazide) Tablets NDA #022314". U.S. Food and Drug Administration (FDA). 2 September 2009. Retrieved 19 September 2021.
External links
- "Amlodipine mixture with valsartan". Drug Information Portal. U.S. National Library of Medicine.
- "Amlodipine besylate mixture with hydrochlorothiazide and valsartan". Drug Information Portal. U.S. National Library of Medicine.
