In chemistry, alpha elimination refers to particular types of elimination reactions. The definition of alpha elimination differs for organometallic and organic chemistry.
Organic chemistry
In organic chemistry, alpha-elimination refers to reactions of this type:[1]
- R2CHX → R2C: + HX
The reaction is employed to generate carbenes and nitrenes. The formation of dichlorocarbene from chloroform is an example.
Alpha eliminations contrasts with beta eliminations, which are commonly used to generate alkenes:
- R2CHCXR'2 → R2C=CR'2 + HX
Both alpha- and beta-eliminations typically require strong base.
Organometallic chemistry
In organometallic chemistry, alpha elimination refers to reactions of this type (other spectator ligands omitted):[2]
- X-M-CH2R → M=CHR + HX
Well studied case are found in organotantalum chemistry leading to an alkylidene derivatives. Specifically, tetraalkyl-monochloro-tantalum complex undergoes α-hydrogen elimination, followed by alkylation of the remaining chloride to give a derivative with a Ta=C bond.[3]
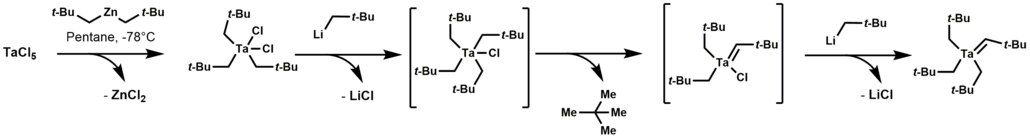
Alpha elimination contrasts with β-hydride elimination, whereby an alkyl group bonded to a metal centre is converted into the corresponding metal-bonded hydride and an alkene.
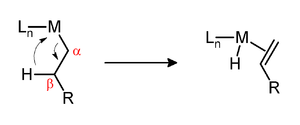
Both α- and β-eliminations proceed via agostic intermediates.[4]
See also
- Elimination reaction, mainly focused on organic substrates.
References
- ^ Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, p. 1477, ISBN 978-0-471-72091-1
- ^ Elschenbroich, C. (2006). Organometallics. Weinheim: Wiley-VCH. ISBN 978-3-527-29390-2.
- ^ Schrock, Richard R. (1979-03-01). "Alkylidene complexes of niobium and tantalum". Accounts of Chemical Research. 12 (3): 98–104. doi:10.1021/ar50135a004. ISSN 0001-4842.
- ^ Brookhart, M.; Green, M. L. H.; Parkin, G. (2007). "Agostic interactions in transition metal compounds". Proceedings of the National Academy of Sciences. 104 (17): 6908–6914. doi:10.1073/pnas.0610747104. PMC 1855361. PMID 17442749.
