 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
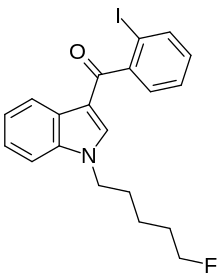
| Formula | C20H19FINO |
| Molar mass | 435.281 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
AM-694 (1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole) is a designer drug that acts as a potent and selective agonist for the cannabinoid receptor CB1. It is used in scientific research for mapping the distribution of CB1 receptors.[1]
YouTube Encyclopedic
-
1/1Views:3 080
-
Hash TEST FAKE Hash Warning video EZTEST
Transcription
Pharmacology
AM-694 is an agonist for cannabinoid receptors. It has a Ki of 0.08 nM at CB1 and 18 times selectivity over CB2 with a Ki of 1.44 nM.[2] It is unclear what is responsible for this unusually high CB1 binding affinity, but it makes the 18F radiolabelled derivative of AM-694 useful for mapping the distribution of CB1 receptors in the body.[1]
Metabolism
Pathways of metabolism include hydrolytic defluorination, carboxylation, and monohydroxylation of the N-alkyl chain.[3][4]
See also
- AM-679
- AM-1235
- AM-2201
- AM-2232
- AM-2233
- FUBIMINA
- JWH-018
- List of AM cannabinoids
- List of JWH cannabinoids
- THJ-2201
References
- ^ a b Willis PG, Katoch-Rouse R, Horti AG (August 2003). "Regioselective F-18 radiolabeling of AM694, a CB1 cannabinoid receptor ligand". Journal of Labelled Compounds and Radiopharmaceuticals. 46 (9): 799–804. doi:10.1002/jlcr.720.
- ^ WO patent 200128557, Makriyannis A, Deng H, "Cannabimimetic indole derivatives", granted 2001-06-07
- ^ Grigoryev A, Kavanagh P, Melnik A (February 2013). "The detection of the urinary metabolites of 1-[(5-fluoropentyl)-1H-indol-3-yl]-(2-iodophenyl)methanone (AM-694), a high affinity cannabimimetic, by gas chromatography - mass spectrometry". Drug Testing and Analysis. 5 (2): 110–5. doi:10.1002/dta.1336. PMID 22522907.
- ^ Apirakkan O, Gavrilović I, Cowan DA, Abbate V (July 2020). "In Vitro Phase I Metabolic Profiling of the Synthetic Cannabinoids AM-694, 5F-NNEI, FUB-APINACA, MFUBINAC, and AMB-FUBINACA". Chemical Research in Toxicology. 33 (7): 1653–1664. doi:10.1021/acs.chemrestox.9b00466. PMID 32301604. S2CID 215803607.
