| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,4-Dioxine[1] | |||
| Systematic IUPAC name
1,4-Dioxacyclohexa-2,5-diene | |||
| Other names
1,4-Dioxin
Dioxin p-Dioxin 1,4-Dioxa[6]annulene | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C4H4O2 | |||
| Molar mass | 84.07 g/mol | ||
| Appearance | Colorless liquid | ||
| Boiling point | 75 °C (167 °F; 348 K) | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards
|
highly flammable | ||
| Related compounds | |||
Related compounds
|
1,2-dioxin, dibenzodioxin | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
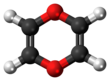
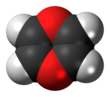
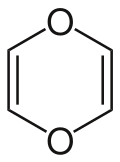
1,4-Dioxin (also referred as dioxin or p-dioxin) is a heterocyclic, organic, non-aromatic[2] compound with the chemical formula C4H4O2. There is an isomeric form of 1,4-dioxin, 1,2-dioxin (or o-dioxin). 1,2-Dioxin is very unstable due to its peroxide-like characteristics.
The term "dioxin" is most commonly used for a family of derivatives of dioxin, known as polychlorinated dibenzodioxins (PCDDs).
YouTube Encyclopedic
-
1/3Views:8 26763025 544
-
How dangerous is dioxane in your drinking water?
-
Dioxin ll Toxic chemical ll sex issue
-
Counteracting the Effects of Dioxins Through Diet
Transcription
How dangerous is the chemical 1,4 dioxane if it gets into water? It may seem an obscure question but if you live somewhere where there's dioxane contamination it's an important one 1,4 dioxane, let's call it dioxane for short is a colorless, flammable liquid that's used as an industrial stabilizer and solvent it dissolves readily in water and doesn't easy biodegrade which is potentially a problem if it gets into the environment and to make matters worse it's considered a probable human carcinogen by the US Environmental Protection Agency. All of this is not good news if stuff somehow gets into your water supply but just how worried should you be if your supply's affected? According to toxicology studies, dioxane --not to be confused with the similarly named but chemically different "dioxin"-- can damage the liver and kidneys if consumed in large quantities. However, an adults would need to be drinking couple microliters of the stuff everyday, or around half thousandth of a teaspoon, for serious harm to occur. This is for non-cancer health risks though. When it comes to cancer things look somewhat different. Based on animal studies the US EPA estimates that if an adult spends their life drinking water contaminated with just three and a half micrograms of dioxane for every liter of water that's less than 1 millionth of a teaspoon they have a one in 100,000 chance of developing liver cancer. But what does this mean in reality? The city of Ann Arbor provides a useful case study here for making practical sense of a somewhat academic risk assessment. From the 1960's to the 1980's tens of thousands of gallons of dioxane were released into the environment to the west to the city. Aquifers underneath the city were contaminated and some dioxane ended up in private wells. Clean-up operations are still ongoing and there is a groundwater use prohibition zone where contamination levels are excessive. There's also a fear that the contamination could reach the city's major water source--a local river--in the future and over the past few decades some people will have been exposed to the chemical. The Michigan cleanup limit for dioxane is 85 parts-per-billion or just under 20 millionth of a teaspoon per liter of water. This is the theoretical level of contamination people could be exposed to before action is taken. So, useful question is what's the cancer risk at this level? Following the US EPA analysis, if you were to drink water contaminated with dioxane at 85 parts-per-billion your whole life your chances of developing liver cancer would be around one in four thousand. This sounds high, but it's based on some assumptions that may not be realistic. Such as assuming that affected adults will be drinking two liter of contaminated water every day of their life seventy years. So what if we consider a more realistic scenario. Imagine for instance that you've lived in Ann Arbor for twenty years and only drink one liter a day of tap water. Your highest dioxin related cancer risk in this case is closer to one in 30,000. Still high but much lower than the one in four thousand. Or imagine you're a teenager who drank 10 liters a week of contaminated water ten years before leaving for college. According to current research your cancer risk would be closer to one in 35,000. These calculations do need to be treated with some care. That's still uncertainty over how 1,4-dioxane causes cancer in animals and the human risk estimates here do err on the side caution. But the do show how a detailed scientific assessment can be used to put a number on real world risks. However, just being able to put a number on the probability of something bad happening does raise some more challenging questions, including what you do with that number once you have it and who decides how much risk is OK especially if someone else created the risk in the first place. These though questions for another risk bites and another day. For more information on 1,4-dioxane check out the links below this always please do join the conversation in the comments.
Preparation
1,4-Dioxin can be prepared by cycloaddition, namely by the Diels–Alder reaction of furan and maleic anhydride. The adduct formed has a carbon-carbon double bond, which is converted to an epoxide. The epoxide then undergoes a retro-Diels–Alder reaction, forming 1,4-dioxin and regenerating maleic anhydride.[3]
Derivatives
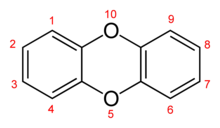
The word "dioxin" can refer in a general way to compounds which have a dioxin core skeletal structure with substituent molecular groups attached to it. For example, dibenzo-1,4-dioxin is a compound whose structure consists of two benzo- groups fused onto a 1,4-dioxin ring.
Polychlorinated dibenzodioxins
Because of their extreme importance as environmental pollutants, current scientific literature uses the name dioxins commonly for simplification to denote the chlorinated derivatives of dibenzo-1,4-dioxin, more precisely the polychlorinated dibenzodioxins (PCDDs), among which 2,3,7,8-tetrachlorodibenzodioxin (TCDD), a tetrachlorinated derivative, is the best known. The polychlorinated dibenzodioxins, which can also be classified in the family of halogenated organic compounds, have been shown to bioaccumulate in humans and wildlife due to their lipophilic properties, and are known teratogens, mutagens, and carcinogens.
PCDDs are formed through combustion, chlorine bleaching and manufacturing processes.[4] The combination of heat and chlorine creates dioxin.[4] Since chlorine is often a part of the Earth's environment, natural ecological activity such as volcanic activity and forest fires can lead to the formation of PCDDs.[4] Nevertheless, PCDDs are mostly produced by human activity.[4]
Famous PCDD exposure cases include Agent Orange sprayed over vegetation by the British military in Malaya during the Malayan Emergency and the U.S. military in Vietnam during the Vietnam War, the Seveso disaster, and the poisoning of Viktor Yushchenko.
Polychlorinated dibenzofurans are a related class compounds to PCDDs which are often included within the general term "dioxins".
References
- ^ "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 147. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Science of Synthesis: Houben-Weyl Methods of Molecular Transformations Vol. 16: Six-Membered Hetarenes with Two Identical Heteroatoms
- ^ Aitken, R. Alan; Cadogan, J. I. G. & Gosneya, Ian (1994). "Effect of ring strain on the formation and pyrolysis of some Diels–Alder adducts of 2-sulfolene (2,3-dihydrothiophene 1,1-dioxide) and maleic anhydride with 1,3-dienes and products derived therefrom". J. Chem. Soc., Perkin Trans. 1 (8): 927–931. doi:10.1039/p19940000927.
- ^ a b c d "Dioxin Information". Department of Environmental Protection, State of Maine. 2005. Archived from the original on 2009-06-15. Retrieved 2008-08-10.