In chemistry, a zwitterion (/ˈtsvɪtəˌraɪən/ TSVIT-ə-rye-ən; from German Zwitter [ˈtsvɪtɐ] 'hermaphrodite'), also called an inner salt or dipolar ion,[1] is a molecule that contains an equal number of positively and negatively charged functional groups.[2] With amino acids, for example, in solution a chemical equilibrium will be established between the "parent" molecule and the zwitterion.
Betaines are zwitterions that cannot isomerize to an all-neutral form, such as when the positive charge is located on a quaternary ammonium group. Similarly, a molecule containing a phosphonium group and a carboxylate group cannot isomerize.
YouTube Encyclopedic
-
1/5Views:624 57237 627426 895304 340932
-
Isoelectric point and zwitterions | Chemical processes | MCAT | Khan Academy
-
Amino Acid: Zwitterion
-
How To Calculate The Isoelectric Point of Amino Acids and Zwitterions
-
Zwitterion and Amino Acid Charge Given pH and pKa
-
Biological Chemistry: What is a zwitterion
Transcription
Hey, so we're going to be talking about the isoelectric point, or pI as it's abbreviated. Now, the isoelectric point is the point along the pH scale at which a molecule, and in this case we're going to be talking about an amino acid, exists in a neutral form with zero charge. In other words, it is neither positively or negatively charged overall. It is isoelectric, and "iso" means equal. And it's nice to know the isoelectric point for an amino acid, because then we can predict whether or not it will be charged at a certain pH. And who doesn't want the power of prediction? So how do we figure out the isoelectric point for an amino acid? Well, let's start with the generic amino acid structure here. So now let's take a look at the two functional groups on this amino acid. Ignoring the R group, or the side chain, for the time being, we're going to be talking about the amino group and the carboxylic acid group. So the amino group here, it has this nitrogen, which is a very happy proton acceptor. So we're going to write that here. And because it's a happy proton acceptor, it is considered to be basic. And we've drawn it out in its protonated form here after it's accepted an extra hydrogen, or proton. So now coming over to our carboxylic acid group, this group is a very willing proton donor. And because it is a proton donor, we call this acidic. And so we've drawn it out here after it's already donated its protons, so it has a negative charge. And now looking at the overall net charge of our amino acid, we can see that we have a positive charge here and a negative charge here, and so the overall charge is 0. And we have a special name for when you have a molecule that has both a positive and a negative charge present. And that special word is called a "zwitterion," which comes from the German word for "hybrid." So now what would happen if we take our amino acid and we put into a solution that is a very low pH, say a pH of 1? In other words, an acidic solution. Well, we can think of acidic solutions as having a lot of excess protons around. So anything that can be protonated on our amino acid is going to be protonated, and so it's going to look like this. And now if you take a look at both of the groups on our amino acid, you can see that our amino group is still in its protonated form and carries a positive charge. But now our carboxylic acid group has gained a proton and lost its negative charge. And now you can see that the overall net charge on this molecule is now positive 1. So now let's come over to the other end of the spectrum. Let's put our amino acid in a solution with a very high pH, say a pH of 12. And so this is going to be really basic solution, and we can think of really basic solutions as having a lot of excess hydroxide anions around. And so now, everything that can be deprotonated on our amino acid will be, so it's going to look like this. And if we look at our overall net charge of our amino acid now, our amino group has been deprotonated so now it is neutral, and the carboxylic acid group has been deprotonated and so it has a negative charge again. And so it has an overall net charge of negative 1. So now we know that we have a range of forms that our amino acid can take. We have the positively charged version at low pHs all the way up to the negatively charged version at high pHs. Now back to our question about the isoelectric point. So the isoelectric point is the pH at which we go from the positive to the negative form. In other words, it's where we find the zwitterion. And to find out the exact pH, we have to take the average of the pKs of our two functional groups. And recall that the pK is just the negative log of the acid dissociation constant. So on average, and it varies between all the different amino acids, but on average, the amino group has a pK of around 9. And then on average, the pK for the carboxylic acid group is right around two. So now if we just give ourselves a little bit more room here, we can calculate what the pI, or isoelectric point, would be for our generic amino acid. So taking the average pK for the amino group and then the average pK for the carboxylic acid group, then we divide by 2, then you get 11 over 2. And we come to an isoelectric point of 5.5. But say our amino acid has a side chain or an R group that is also a functional group? Then, we would also have to take the pK for that group into account when we calculate the isoelectric point. So what have we learned? Well, we've learned that the isoelectric point is the pH at which a molecule's found in neutral form, in this case, when an amino acid is in its zwitterion form. And we also learned how to calculate this isoelectric point for an amino acid by taking the average of the pKs of all the functional groups in that amino acid.
Amino acids

Tautomerism of amino acids follows this stoichiometry:
- RCH(NH2)CO2H ⇌ RCH(N+H3)CO−2
The ratio of the concentrations of the two species in solution is independent of pH.
It has been suggested, on the basis of theoretical analysis, that the zwitterion is stabilized in aqueous solution by hydrogen bonding with solvent water molecules.[3] Analysis of neutron diffraction data for glycine showed that it was in the zwitterionic form in the solid state and confirmed the presence of hydrogen bonds.[4] Theoretical calculations have been used to show that zwitterions may also be present in the gas phase for some cases different from the simple carboxylic acid-to-amine transfer.[5]
The pKa values for deprotonation of the common amino acids span the approximate range 2.15±0.2. This is also consistent with the zwitterion being the predominant isomer that is present in an aqueous solution. For comparison, the simple carboxylic acid propionic acid (CH3CH2CO2H) has a pKa value of 4.88.
Other compounds
-
Sulfamic acid isomers, with the zwitterion (right)
-
-
Structure of H4EDTA
-
Sulfamic acid crystallizes in the zwitterion form.[6]
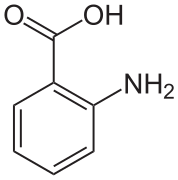
In crystals of anthranilic acid there are two molecules in the unit cell. One molecule is in the zwitterion form, the other is not.[7]
In the solid state, H4EDTA is a zwitterion with two protons having been transferred from carboxylic acid groups to the nitrogen atoms.[8]
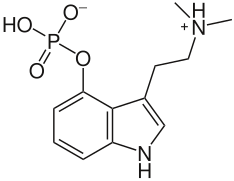
In psilocybin, the proton on the dimethyl amino group is labile and may jump to the phosphate group to form a compound which is not a zwitterion.
Theoretical studies

Insight to the equilibrium in solution may be gained from the results of theoretical calculations. For example, pyridoxal phosphate, a form of vitamin B6, in aqueous solution is predicted to have an equilibrium favoring a tautomeric form in which a proton is transferred from the phenolic -OH group to the nitrogen atom.[9]
Because tautomers are different compounds, they sometimes have different enough structures that they can be detected independently in their mixture. This allows experimental analysis of the equilibrium.[10]
Betaines and similar compounds
The compound trimethylglycine, which was isolated from sugar beet, was named as "betaine". Later, other compounds were discovered that contain the same structural motif, a quaternary nitrogen atom with a carboxylate group attached to it via a –CH2– link. At the present time, all compounds whose structure includes this motif are known as betaines. Betaines do not isomerize because the chemical groups attached to the nitrogen atom are not labile. These compounds may be classed as permanent zwitterions, as isomerisation to a molecule with no electrical charges does not occur, or is very slow.[11]
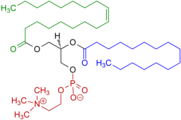
Other examples of permanent zwitterions include phosphatidylcholines, which also contain a quaternary nitrogen atom, but with a negatively-charged phosphate group in place of a carboxylate group; sulfobetaines, which contain a quaternary nitrogen atom and a negatively charged sulfonate group;[12] and pulmonary surfactants such as dipalmitoylphosphatidylcholine. Lauramidopropyl betaine is the major component of cocamidopropyl betaine.
-
Trimethylglycine (trivial name betaine)
-
Example of a phosphatidylcholine
-
Conjugated zwitterions
Strongly polarized conjugated compounds (conjugated zwitterions) are typically very reactive, share diradical character, activate strong bonds and small molecules, and serve as transient intermediates in catalysis.[13] Donor-acceptor entities are of vast use in photochemistry (photoinduced electron transfer), organic electronics, switching and sensing.
See also
References
- ^ "Zwitterion". Chemistry LibreTexts. 2015-11-03. Archived from the original on 2023-06-21. Retrieved 2022-02-11.
- ^ Skoog, Douglas A.; West, Donald M.; Holler, F. James; Crouch, Stanley R. (2004). Fundamentals of Analytical Chemistry (8th ed.). Thomson/Brooks/Cole. pp. 231, 385, 419, 460. ISBN 0-03-035523-0.
- Fundamentals of Analytical Chemistry (9th ed.). 2013. pp. 415–416. ISBN 978-1-285-60719-1.
- ^ Jensen, Jan H.; Gordon, Mark S. (1995). "On the Number of Water Molecules Necessary to Stabilize the Glycine Zwitterion". Journal of the American Chemical Society. 117 (31): 8159–8170. doi:10.1021/ja00136a013. Archived from the original on 2020-12-02. Retrieved 2020-08-28.
- ^ Jönsson, P.-G.; Kvick, Å. (1972). "Precision neutron diffraction structure determination of protein and nucleic acid components. III. The crystal and molecular structure of the amino acid α-glycine" (PDF). Acta Crystallographica Section B. 28 (6): 1827–1833. doi:10.1107/S0567740872005096. Archived (PDF) from the original on 2020-03-14. Retrieved 2019-09-03.
- ^ Price, William D.; Jockusch, Rebecca A.; Williams, Evan R. (1997). "Is Arginine a Zwitterion in the Gas Phase?". Journal of the American Chemical Society. 119 (49): 11988–11989. doi:10.1021/ja9711627. PMC 1364450. PMID 16479267.
- ^ Sass, R. L. (1960). "A neutron diffraction study on the crystal structure of sulfamic acid". Acta Crystallographica. 13 (4): 320–324. doi:10.1107/S0365110X60000789.
- ^ Brown, C. J.; Ehrenberg, M. (1985). "Anthranilic acid, C7H7NO2, by neutron diffraction". Acta Crystallographica C. 41 (3): 441–443. doi:10.1107/S0108270185004206.
- ^ Cotrait, Par Michel (1972). "La structure cristalline de l'acide éthylènediamine tétraacétique, EDTA" [The crystalline structure of ethylenediamine tetraacetic acid, EDTA]. Acta Crystallographica B. 28 (3): 781–785. doi:10.1107/S056774087200319X.
- ^ Kiruba, G. S. M.; Ming, Wah Wong (2003). "Tautomeric Equilibria of Pyridoxal-5′-phosphate and 3-Hydroxypyridine Derivatives: A Theoretical Study of Solvation Effects". Journal of Organic Chemistry. 68 (7): 2874–2881. doi:10.1021/jo0266792. PMID 12662064.
- ^ Nagy, Peter I.; Takács-Novák, Krisztina (1997). "Theoretical and Experimental Studies of the Zwitterion ⇌ Neutral Form Equilibrium of Ampholytes in Pure Solvents and Mixtures". J. Am. Chem. Soc. 119 (21): 4999–5006. doi:10.1021/ja963512f.
- ^ Nelson, D. L.; Cox, M. M. (2000). Lehninger, Principles of Biochemistry (3rd ed.). New York: Worth Publishing. ISBN 1-57259-153-6.
- ^ Gonenne, Amnon; Ernst, Robert (1978-06-15). "Solubilization of membrane proteins by sulfobetaines, novel zwitterionic surfactants". Analytical Biochemistry. 87 (1): 28–38. doi:10.1016/0003-2697(78)90565-1. ISSN 0003-2697. PMID 677454.
- ^ Munz, Dominik; Karsten, Meyer (2021). "Charge frustration in ligand design and functional group transfer". Nat. Rev. Chem. 5 (6): 422–439. doi:10.1038/s41570-021-00276-3. S2CID 235220781.