| Vaterite | |
|---|---|
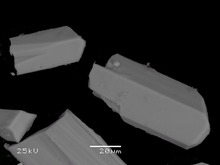 Vaterite from San Vito quarry, San Vito, Monte Somma, Somma-Vesuvius Complex, Italy | |
| General | |
| Category | Carbonate minerals |
| Formula (repeating unit) | CaCO3 |
| IMA symbol | Vtr[1] |
| Strunz classification | 5.AB.20 |
| Crystal system | Hexagonal |
| Crystal class | Dihexagonal dipyramidal (6mmm) H-M symbol: (6/m 2/m 2/m) |
| Space group | P63/mmc {P63/m 2/m 2/c} |
| Unit cell | a = 4.13, c = 8.49 [Å]; Z = 6 |
| Identification | |
| Color | Colorless |
| Crystal habit | Fine fibrous crystals, typically less than 0.1 mm, in spherulitic aggregates. |
| Fracture | Irregular to uneven, splintery |
| Tenacity | Brittle |
| Mohs scale hardness | 3 |
| Luster | Sub-vitreous, waxy |
| Diaphaneity | Transparent to semi-transparent |
| Specific gravity | 2.54 |
| Optical properties | Uniaxial (+) |
| Refractive index | nω = 1.550 nε = 1.650 |
| Birefringence | δ = 0.100 |
| References | [2][3][4] |
Vaterite is a mineral, a polymorph of calcium carbonate (CaCO3). It was named after the German mineralogist Heinrich Vater. It is also known as mu-calcium carbonate (μ-CaCO3). Vaterite belongs to the hexagonal crystal system, whereas calcite is trigonal and aragonite is orthorhombic.
Vaterite, like aragonite, is a metastable phase of calcium carbonate at ambient conditions at the surface of the Earth. As it is less stable than either calcite or aragonite, vaterite has a higher solubility than either of these phases. Therefore, once vaterite is exposed to water, it converts to calcite (at low temperature) or aragonite (at high temperature: ~60 °C). At 37 °C for example a solution-mediated transition from vaterite to calcite occurs, where the vaterite dissolves and subsequently precipitates as calcite assisted by an Ostwald ripening process.[5]
However, vaterite does occur naturally in mineral springs, organic tissue, gallstones, urinary calculi and plants. In those circumstances, some impurities (metal ions or organic matter) may stabilize the vaterite and prevent its transformation into calcite or aragonite. Vaterite is usually colorless.
Vaterite can be produced as the first mineral deposits repairing natural or experimentally-induced shell damage in some aragonite-shelled mollusks (e.g. gastropods). Subsequent shell deposition occurs as aragonite. In 2018, vaterite was identified as a constituent of a deposit formed on the leaves of Saxifraga at Cambridge University Botanic Garden.[6][7]
Vaterite has a JCPDS number of 13-192.


YouTube Encyclopedic
-
1/3Views:602860580
-
Aragonite
-
Otolith
-
Protein nucleation and crystal growth
Transcription
See also
References
- ^ Warr, L.N. (2021). "IMA–CNMNC approved mineral symbols". Mineralogical Magazine. 85 (3): 291–320. Bibcode:2021MinM...85..291W. doi:10.1180/mgm.2021.43. S2CID 235729616.
- ^ Mindat.org
- ^ Handbook of Mineralogy
- ^ Webmineral data
- ^ Zhou, Gen-Tao; Yao, Qi-Zhi; Fu, Sheng-Quan; Guan, Ye-Bin (2010). "Controlled crystallization of unstable vaterite with distinct morphologies and their polymorphic transition to stable calcite". European Journal of Mineralogy. 22 (2): 259–269. Bibcode:2010EJMin..22..259Z. doi:10.1127/0935-1221/2009/0022-2008.
- ^ Chris Elliott (12 Mar 2018). "Incredible discovery at Cambridge's Botanic Garden that could transform treatment of cancer". Cambridge News. Archived from the original on 2018-03-12.
- ^ "Rare mineral discovered in plants for first time". Science Daily. 5 Mar 2018.
