| |
| Names | |
|---|---|
| Other names
* N,N-Bis(carboxymethyl)-DL-alanine trisodium salt
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.120.943 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H8NNa3O6 | |
| Molar mass | 271.111 g·mol−1 |
| Density | * 0.690 g/cm3[1] as powder |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H290 | |
| P234, P390, P404 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Trisodium N-(1-carboxylatoethyl)iminodiacetate, methylglycinediacetic acid trisodium salt (MGDA-Na3) or trisodium α-DL-alanine diacetate (α-ADA), is the trisodium anion of N-(1-carboxyethyl)iminodiacetic acid and a tetradentate complexing agent. It forms stable 1:1 chelate complexes with cations having a charge number of at least +2, e.g. the "hard water forming" cations Ca2+ or Mg2+. α-ADA is distinguished from the isomeric β-alaninediacetic acid by better biodegradability and therefore improved environmental compatibility.[3]
Production
The patent literature on the industrial synthesis of trisodium N-(1-carboxylatoethyl)iminodiacetate describes the approaches for solving the key requirements of a manufacturing process that can be implemented on an industrial scale, characterized by
- Achieving the highest possible space-time yields
- Simple reaction control at relatively low pressures and temperatures
- Realization of continuous process options
- Achieving the lowest possible levels of impurities, particularly nitrilotriacetic acid, which is suspected of being carcinogenic
- Use of inexpensive raw materials, e.g. instead of pure L-alanine the raw mixture of Strecker synthesis from methanal, hydrogen cyanide and ammonia
- Avoidance of complex and yield-reducing isolation steps; instead, direct further use of the crude reaction solutions or precipitates in the following process step.
An obvious synthesis route to α-alaninediacetic acid is from racemic α-DL-alanine, which provides racemic α-ADA by double cyanomethylation with methanal and hydrogen cyanide, hydrolysis of the intermediately formed diacetonitrile to the trisodium salt and subsequent acidification with mineral acids in a 97.4% overall yield.[4] In a later patent specification, however, only an overall yield of 77% and an NTA content of 0.1% is achieved with practically the same quantities of substances and under practically identical reaction conditions.[5]
This later patent specification[5] also indicates a process route via alaninonitrile, which is obtained by Strecker synthesis from hydrogen cyanide, ammonia and methanal and converted to methylglycinonitrile-N,N-diacetonitrile by double cyanomethylation (step 1). The three nitrile groups are then hydrolyzed with sodium hydroxide to α-ADA (step 2). The total yield is given as 72%, the NTA content as 0.07%.
One variant of the reaction involves iminodiacetonitrile or iminodiacetic acid (step 1'), which reacts in a weakly acidic medium (pH 6) with hydrogen cyanide and ethanal to form methylglycinonitrile-N,N-diacetic acid, the nitrile group of which is hydrolyzed with sodium hydroxide to trisodium N-(1-carboxylatoethyl)iminodiacetate (step 2'). The reactant iminodiacetic acid is accessible at low cost by dehydrogenation of diethanolamine. Again, the total yield is given as 72%, the NTA content as 0.07%.
A further variant is suitable for continuous production, in which ammonia, methanal and hydrogen cyanide react at pH 6 to form iminodiacetonitrile, which in a strongly acidic medium (pH 1.5) reacts with ethanal to produce trinitrile methylglycinonitrile-N,N-diacetonitrile in a very good yield of 92%. (step 1).
Alkaline hydrolysis (step 2) results in a total yield of 85% trisodium N-(1-carboxylatoethyl)iminodiacetate with an NTA content of 0.08%. This process variant seems to fulfil the above-mentioned criteria best.
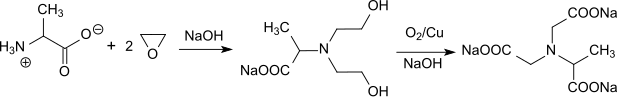
A low by-product synthesis route for trisodium N-(1-carboxylatoethyl)iminodiacetate has recently been described, in which alanine is ethoxylated with ethylene oxide in an autoclave to form bis-hydroxyethylaminoalanine and then oxidized to α-ADA at 190 °C with Raney copper under pressure.[6]
The yields are over 90% d.Th., the NTA contents below 1%. The process conditions make this variant rather less attractive.
Properties
The commercially available trisodium N-(1-carboxylatoethyl)iminodiacetate (84% by weight) is a colourless, water-soluble solid whose aqueous solutions are rapidly and completely degraded even by non-adapted bacteria. Aquatic toxicity to fish, daphnia and algae is low.[7] Trisodium N-(1-carboxylatoethyl)iminodiacetate is described as readily biodegradable (OECD 301C) and is eliminated to >90 % in wastewater treatment plants.[8] Trisodium N-(1-carboxylatoethyl)iminodiacetate has not yet been detected in the discharge of municipal and industrial sewage treatment plants. In addition to their very good biodegradability, trisodium N-(1-carboxylatoethyl)iminodiacetate solutions are characterized by high chemical stability even at temperatures above 200 °C (under pressure) in a wide pH range between 2 and 14 as well as high complex stability compared to other complexing agents of the aminopolycarboxylate type.[1][9]
The following table shows the complexing constants log K of α-ADA compared to tetrasodium iminodisuccinate and ethylenediaminetetraacetic acid (EDTA) versus polyvalent metal ions:
| Metal ions | MGDA | IDS[10] | EDTA[11] |
|---|---|---|---|
| Ba2+ | 4.9 | 3.4 | 7.9 |
| Ag+ | 3.9 | 7.3 | |
| Sr2+ | 5.2 | 4.1 | |
| Ca2+ | 7.0 | 5.2 | 10.6 |
| Mg2+ | 5.8 | 6.1 | 8.7 |
| Mn2+ | 8.4 | 7.7 | 13.8 |
| Fe2+ | 8.1 | 8.2 | 14.3 |
| Cd2+ | 10.6 | 8.4 | 16.5 |
| Cr3+ | 9.6 | ||
| Co2+ | 11.1 | 10.5 | 16.3 |
| Zn2+ | 10.9 | 10.8 | 16.5 |
| Pb2+ | 12.1 | 11.0 | 18.0 |
| Ni2+ | 12.0 | 12.2 | 18.6 |
| Cu2+ | 13.9 | 13.1 | 18.8 |
| Al3+ | 14.1 | 16.1 | |
| Hg2+ | 14.9 | 21.8 | |
| Fe3+ | 16.5 | 15.2 | 25.1 |
The complex formation constants of the biodegradable chelators α-ADA and IDS are in a range suitable for industrial use, but clearly below those of the previous standard EDTA.
In solid preparations, trisodium N-(1-carboxylatoethyl)iminodiacetate is stable against oxidizing agents such as perborates and percarbonates, but not against oxidizing acids or sodium hypochlorite.
Use
Like other complexing agents in the aminopolycarboxylic acid class, trisodium N-(1-carboxylatoethyl)iminodiacetate (α-ADA) finds due to its ability to form stable chelate complexes with polyvalent ions (in particular the water hardening agents Ca2+ and Mg2+, as well as transition and heavy metal ions such as Fe3+, Mn2+, Cu2+, etc.) use in water softening, in detergents and cleaning agents, in electroplating, cosmetics, paper and textile production. Due to its stability at high temperatures and pH values, α-ADA should be particularly suitable as a substitute for the phosphates banned in the EU from 2017, such as sodium tripolyphosphate (STPP)[12] in tabs for dishwashers.
BASF SE is the most important manufacturer of α-ADA under the brand name Trilon M, has large-scale plants in Ludwigshafen and Lima, Ohio, and is currently expanding its existing capacities with another large-scale plant at Evonik's site in Theodore, Alabama.[13]
References
- ^ a b c BASF SE, Technical Information: Trilon M Types Archived 2019-07-13 at the Wayback Machine
- ^ National Industrial Chemicals Notification and Assessment Scheme (NICNAS): Full Public Report "Methyl glycine diacetic acid, trisodium salt", File No: STD/1092, August 2004.
- ^ Environmental Protection Agency, DfE's Safer Chemical Ingredients List, Chelating Agents, Alanine, N,N-bis(carboxymethyl)-, sodium salt (1:3).
- ^ WO 9429421, J. Schneider et al., "Use of glycine-N,N-diacetic acid derivatives as biodegradable complexing agents for alkaline earth metal ions and heavy metal ions, and methods of preparing them", issued 1994-12-22, assigned to BASF AG
- ^ a b US 5849950, T. Greindl et al., "Preparation of glycine-N,N-diacetic acid derivatives", issued 1998-12-15, assigned to BASF AG
- ^ EP 2547648, R. Baumann et al., "Verfahren zur Herstellung nebenproduktarmer Aminocarboxylate", issued 2013-01-23, assigned to BASF SE
- ^ BASF, Sicherheitsdatenblatt: Trilon M Powder MSDS
- ^ Hessisches Landesamt für Umwelt und Geologie, 6.12 Komplexbildner. 2003, S. 12/6.
- ^ Kołodyńska, Dorota; Hubicka, Halina; Hubicki, Zbigniew (2009). "Studies of application of monodisperse anion exchangers in sorption of heavy metal complexes with IDS". Desalination. 239 (1–3): 216–228. Bibcode:2009Desal.239..216K. doi:10.1016/j.desal.2008.02.024..
- ^ Lanxess AG, General Product Information: Baypure
- ^ BASF SE, Technical Information: Trilon B Types (Dec 2013)
- ^ SEPAWA, Rückblick 2013, Abstracts: Wasch- und Reinigungsmittel Session Reinigen und Hygiene, Jürgen Kielholz: Phosphatfreie Reiniger für maschinelle Geschirrspüler Archived 2014-07-14 at the Wayback Machine
- ^ BASF SE: No more tea stains and chalky deposits[permanent dead link]