| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
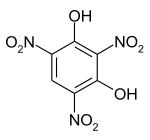
2,4,6-Trinitrobenzene-1,3-diol | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.001.306 | ||
PubChem CID
|
|||
| UNII | |||
| UN number | 0219 – Dry or wetted with < 20% water/alcohol 0394 – Wetted with >= 20% water/alcohol | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H3N3O8 | |||
| Molar mass | 245.11 g/mol | ||
| Density | 1.829 g/cm3 | ||
| Melting point | 180 °C (356 °F; 453 K) | ||
| Boiling point | decomposes | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Styphnic acid (from Greek stryphnos "astringent"[1]), or 2,4,6-trinitro-1,3-benzenediol, is a yellow astringent acid that forms hexagonal crystals. It is used in the manufacture of dyes, pigments, inks, medicines, and explosives such as lead styphnate. It is itself a low-sensitivity explosive, similar to picric acid, but explodes upon rapid heating.[2]
YouTube Encyclopedic
-
1/2Views:42110 863
-
Carboxylation of resorcinol
-
Lead Styphnate detonation
Transcription
Preparation and chemistry
It may be prepared by the nitration of resorcinol with a mixture of nitric and sulfuric acid.[3]
This compound is an example of a trinitrophenol.
Like picric acid, it is a moderately strong acid, capable of displacing carbon dioxide from solutions of sodium carbonate, for example.
It may be reacted with weakly basic oxides, such as those of lead and silver, to form the corresponding salts.
The solubility of picric acid and styphnic acid in water is less than their corresponding mono- and di-nitro compounds, and far less than their non-nitrated precursor phenols, so they may be purified by fractional crystallisation.
References
- ^ Alexander Senning (2006). Elsevier's Dictionary of Chemoetymology: The Whys and Whences of Chemical Nomenclature and Terminology, p. 375, at Google Books
- ^ Armarego, W.L.F.; Chai, C.L.L. (2003). Purification of Laboratory Chemicals. Butterworth-Heinemann. p. 353. ISBN 9780750675710. Retrieved 2015-05-20.
- ^ Barros, Sam. "PowerLabs Styphnic Acid Synthesis!". powerlabs.org. Retrieved 2015-05-20.