| |||
| Names | |||
|---|---|---|---|
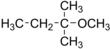
| Preferred IUPAC name
2-Methoxy-2-methylbutane | |||
| Other names
tertiary-Amyl methyl ether; TAME; Methoxypentane
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| Abbreviations | TAME | ||
| ChemSpider | |||
| ECHA InfoCard | 100.012.374 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H14O | |||
| Molar mass | 102.177 g·mol−1 | ||
| Appearance | Clear, colorless liquid | ||
| Density | 0.76-0.78 g/mL[3] | ||
| Melting point | −80 °C (−112 °F; 193 K) | ||
| Boiling point | 86.3 °C (187.3 °F; 359.4 K) | ||
| 10.71 g/L at 20 °C | |||
Refractive index (nD)
|
1.3896 | ||
| Hazards | |||
| Flash point | −11 °C (12 °F; 262 K) | ||
| 430 °C (806 °F; 703 K) | |||
| Explosive limits | 1.0-7.1% | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
tert-Amyl methyl ether (TAME) is an ether used as a fuel oxygenate. TAME derives from C5 distillation fractions of naphtha.[4] It has an ethereous odor.[1] Unlike most ethers, it does not require a stabilizer as it does not form peroxides on storage.[5]
Other names:[6]
- 2-Methoxy-2-methylbutane
- Butane, 2-methoxy-2-methyl-
- 1,1-Dimethylpropyl methyl ether
- Methyl tert-pentyl ether
- Methyl tert-Amyl ether
- 2-Methyl-2-methoxybutane
- Methyl 2-methyl-2-butyl ether
- tert-Pentyl methyl ether
- Tertiary amyl methyl ether
- Methyl 1,1-dimethylpropyl ether
- 2-Methoxy-2-methylbutane
YouTube Encyclopedic
-
1/3Views:596 0068 27231 270
-
Common and systematic naming: iso-, sec-, and tert- prefixes | Organic chemistry | Khan Academy
-
361L Dehydration of 2-methyl-2-butanol (#9)
-
How To Identify Branched Substituents like Isopropyl, Isobutyl, Sec Butyl, Tertbutyl, Isopentyl
Transcription
Let's see if we can get the molecular structure for butylcyclopentane. So you just break this up the way we've done it in the last several videos, the suffix is -ane, so it is an alkane, all single bonds. So single bonds. It's pentane, so we're dealing with five carbons on the base, or on the backbone. So this is five carbons and it's a cyclopentane, so it's five carbons in a ring. So its five-carbon ring is the backbone, and then we have a butyl group added to that five-carbon ring. Now, you might say, hey, Sal, how do I know which carbon to add it to? When you're dealing with a ring and you only have one group on the ring, it doesn't matter. Let me just show you what I mean. So let's draw the five-carbon ring. Let's draw the cyclopentane. So it'll just be a pentagon, so one, two, three, four, five, and it's a ring, so you can connect them. One, two, three, four, five. Now, it doesn't matter where I draw the butyl group. It's all symmetric around there. We just have a ring and it's connected to a butyl group at some point. It'll start to matter once we add more than one group. So we can just pick any of these carbons to add the butyl group to. Now, just as a review, the but- prefix, that refers to, remember, methyl, ethyl, propyl, or meth-, eth-, prop-, but-. This is four carbons. This is a four-carbon alkyl group. So let me just add it here. I could have added it to any of these carbons around this cyclopentane ring. So if I just add it right here, so I'm going to have four carbons. So one, two, three, four. That is the butyl part of this whole thing. And then let me just attach them up. So you might be tempted to just draw this right there. And actually, this would be right. This is butylcyclopentane. But a question might arise. I just happened to connect the cyclopentane to the butyl at this first carbon on the butyl right there. I could have just as easily done it like this. I could have just as easily had it like this, where-- let me draw my butyl again, so I have one, two, three, four. So, once again, this is a butyl, but instead of being bonded to the cyclopentane on my first carbon, maybe it's bonded right here. Let me do it with that yellow color. Maybe it's bonded right here. This seems like maybe this could also be butylcyclopentane. It looks like we have a butyl group. This is a butyl right here. I drew a butyl group right over here, and I also drew a butyl group right over here. But these are fundamentally two different molecular structures. I'm touching the first carbon here. I'm touching the second carbon over here. Now, there's two ways to differentiate this. One is the common naming and one is the systematic naming. So let me differentiate between the two. So in the common naming, and this can get a little bit involved, and this frankly is probably the most complicated part of naming organic compounds. Systematic is often more complicated, but it's easier to systematically come up with it. So there's a common and then there's a systematic. So the common way of doing it is, if you just say butylcyclopentane, that implies that you are bonding to the first or, depending on how you view it, the last carbon in the chain. So this right here is butylcyclopentane. This right here is not just butylcyclopentane. What you would do is you definitely have a cyclopentane ring, so this would definitely be a cyclopentane. Let me put some space here. This is definitely going to be a cyclopentane. And you do have a butyl group on it, so we do have a butyl group, but because we are bonded-- we aren't bonded to the first carbon. We're bonded to a carbon that is bonded to two other carbons. We call this sec-butylcyclopentane, so this is sec-. And everything I'm doing is obviously free-hand. If you were to see this in a book, the sec- would be italicized, or sometimes it would be written as s-butylcyclopentane. And this sec- means that we have attached to a carbon that is touching two other carbons. So you look at the butyl group, and say, well, which of these carbons is attached to two others? It's either that one or that one. And regardless of whether you're attached to this or this, if you think about it, it's fundamentally the same molecular structure. So that's what you do when you're attached to that guy right over there. But what about the situation where we're dealing with just the common names right here? What about the situation where it looks like this? So we have our cyclopentane right there, and we have a-- I guess we could call it a butyl group. It'll have four carbons in it, but let's say that the four carbons look something like this. Let's say there are four carbons, so we have one, two, three, four carbons and we're bonded to this one right over here. So whenever you're bonded to one end of the four-carbon group and it branches off at the other end, and it seems a little complicated, this only deals for alkyl groups below five or six carbons, this we call an isobutyl group. So let me write this down. So this right here, is sec-butyl, or s-butyl sometimes for short. This right here, this right there is iso-butyl. It's actually-- iso- that is an iso-butyl group. And then the last thing to worry about when you're dealing with butyl groups is something like this. Let me draw it. So you could also draw four carbons like this. You have one carbon, two, three, four. One, two, three, four carbons and you're attached over here. Now this naming, this group right here-- and you're going see the systematic naming is much easier for these compounds. This group right here, over here the carbon you're attached to is attached to two other carbons, so it is sec-butyl. When you're attached to three carbons, it is t-butyl, or tert-butyl, so this right here is a tert-butyl group, or sometimes called a t-butyl. And I really want you to understand the difference here. The common naming, it's easier to say and easier to spell, but it's sometimes a little confusing. This is just straight up butyl so you would call this butylcyclopentane. This is sec-butyl, because you have this guy connected to two carbons. That's where the sec- comes from. Sometimes it'll be s-butyl. So this could be called sec-butylcyclopentane or s-butylcyclopentane. This, because we're attached to the end away from this branching off, is still a butyl group, since we have four carbons. But since we're attached here, this is iso-butyl, so this is iso-butylcyclopentane, And then finally, since the carbon we're attaching to is attached to one, two, three other carbons, it is a tert-butyl or a t-butyl group. So this is t-butylcyclopentane. That's the common naming. So maybe I should clear out systematic here just so it's clear to you that everything we've done here is common naming. So let me write it down. It won't hurt to write them down again because the more familiar you are with these, the better. So this is just butylcyclopentane. This is s- or sec- butylcyclopentane. And this is iso-butylcyclopentane. I'm going off the screen here. And then finally, this is tert-butyl, or t-butylcyclopentane. Now, I said these are the common naming. What are the systematic naming? Well, in the systematic, this is still butylcyclopentane. So let me write this down. Systematic, this is still butylcyclopentane, which makes sense. This is very clearly a cyclopentane. This is very clearly a butyl group. But in the systematic naming, what we try to do is we try to name this group right here just as we would name a traditional chain, but we ended it with an -yl. So if you look at this right here, what we do is we just consider the chain where we attach. We attached over here, so the longest chain from that point is there and there. So if you look at it like that, it looks like you have one, two, three carbons, and you have one carbon attached on the beginning. So this little group right here in the systematic naming, this looks like a one, two, three. Three carbons, that's the prop- prefix, so we're dealing with a prop-, and it's all going to be one group, so it's a propyl group. This is a propyl group, but it has a methyl-- remember, meth- is one carbon. It has a methyl group attached on the first carbon. So this is 1-methylpropyl. Now, that describes just the group. 1-methylpropyl describes just this part right here. That describes just that right over there. And then to have the whole compound, to describe the whole compound, you put this in parentheses, so this is the systematic naming. So 1-methyl-- I put an L there. Let me do it in the same color. 1-methyl, because you're starting where you're attaching. So 1-methyl, you have a methyl group right there on that first carbon. It's a propyl chain. One, two, three, propyl, and then you would say cyclopentane. That's the systematic name for that. Now if you look at this one right here, and the common name is iso-butyl, what you do is you look at where we attach. Where we attached is one, two, three carbons. So once again, I'm doing that same one, two, three carbons. So once again, this is a propyl. Prop- is for three, but with a methyl group now is attached to the one, two, the second carbon. So this is 2-methyl. Let me make some space here. This is 2-methyl. So that describes this group right here. That describes this entire group cyclopentane. Remember, this is a systematic name. You might sometimes see this referred to as iso-butylcyclopentane or 2-methylpropylcyclopentane. And this is actually a -yl. I spelled it wrong. And then finally, we do the same exact idea here, but it becomes a little bit more interesting. Over here, we are attached to this carbon and the longest chain I can do starting with that carbon is just one chain right there. So we just have a two-carbon chain, right? One, two. The prefix for a two carbon is ethyl, or eth-. Eth-, and since it's a group, ethyl. And then we have two methyl groups attached right over there, and it's attached on the one carbon, right? This is one and this is two. We call the one carbon where we are attached to the broader chain. So what this is going to be, you would actually write one comma one to show that we have two groups attached to the first carbon and both of them are methyl. So we write 1,1-dimethyl, di- for two, dimethyl. So this entire group right here, which we also called t-butyl, in systematic naming is 1,1-. We have two groups attached to this first carbon. 1,1-dimethylethyl. That's this whole thing. This is the ethyl, and then we have two methyls attached there. that's why we write 1,1-. They're both attached to the one. We have two of them. That's why we wrote di- over there. So it's 1,1-dimethylethyl- and then finally, cyclopentane. So hopefully, that doesn't confuse you too much. I think if you watch the video over and over and try to practice it with your own problems, you'll see that the systematic name way is actually pretty, pretty logical. And actually, if you have more than five or six carbons in the group, they always or they tend to always use the systematic naming.
Uses
TAME is mostly used as an oxygenate to gasoline. It is added for three reasons: to increase octane enhancement, to replace banned tetraethyl lead, and to raise the oxygen content in gasoline. It is known that TAME in fuel reduces exhaust emissions of some volatile organic compounds.[1]
TAME is also used as a solvent in organic synthesis as a more environmentally friendly alternative to some of the classic ether solvents.[4] It is characterized by a high boiling point (86°C) and a low freezing point (−80°C), allowing a wide range of reaction temperatures. TAME can be used as a safe reaction medium (e.g. condensation reactions, coupling reactions, such as Grignard reactions and Suzuki reactions, as well as metal hydride reductions) and as an extraction solvent to replace dichloromethane, aromatics, and other ethers.[7][failed verification]
A series of experiments were carried out in a batch reactor at the temperature range of 313-343 K to study the synthesis of tert-amyl ethyl ether from ethanol (EtOH) and 2-methyl-1-butene (2M1B) catalyzed by the NKC-9 ion-exchange resin. The suitable reaction pressure was obtained by using the method of the Gibbs free energy minimization. The activity coefficients of each component were accurately calculated using the Wilson method, then, the equilibrium constants was obtained. The effect of catalyst size, stirring rate, temperature and EtOH/2M1B molar ratio was investigated at the chosen pressure, respectively. A kinetic model which considered the variation of each component volume was established. The method of nonlinear least square combined with genetic algorithm (NLS-GA) was proposed to estimate the kinetic constant in the forward direction. Results indicated that simulated kinetics results were agreed well with the experimental data.
Toxicity
TAME was evaluated in 4-week rat inhalation studies sponsored by Amoco Corporation. Target vapor concentrations were 0, 500, 2000, or 4000 ppm for 6 h per day, 5 days per week, for 4 weeks. Exposure at 4000 ppm resulted in 25% mortality, apparently as a consequence of severe CNS depression. Body weight gain was decreased in the TAME high dose male rats. Significant effects on functional observational battery (FOB) parameters were only found in the high and mid-dose groups immediately after exposure. All affected FOB parameters were normal by the next day. TAME exposure significantly increased relative liver weights in the high and mid-dose groups. However, no treatment-related histopathologic findings were noted for the compound. Clinical chemistry and hematology findings were minimal with TAME exposure. The results indicate that 500 ppm was a NOAEL for TAME in these studies.[8]
Some other properties[6]
Relative vapor density (air = 1): 3.6
Vapor Pressure 75.2 [mmHg]
log Kow = 1.55 at 20 °C
Henry's Law constant = 1.32X10-3 atm-cu m/mol at 25 °C
Stability / Shelf Life: Stable under recommended storage conditions.
Autoignition Temperature: 415 °C
Decomposition: When heated to decomposition it emits acrid smoke and irritating vapors.
Odor Threshold: 0.02 [mmHg]
Kovats retention index
Standard non-polar 672.5, 674, 673, 669.3, 666
Semi-standard non-polar 678, 655, 668.3
Standard polar 790, 802.9
See also
References
- ^ a b c "tert-AMYL METHYL ETHER (1,1-DIMETHYLPROPYL METHYL ETHER)". chemicalland21.com. Retrieved 2009-10-20.
- ^ National Industrial Chemicals Notification and Assessment Scheme (2001). "t-Amyl methyl ether (TAME)" (PDF). Full Public Reports. Retrieved 2009-10-20.
- ^ "tert-Amyl methyl ether". Sigma-Aldrich.
- ^ a b Prat, Denis; Wells, Andy; Hayler, John; Sneddon, Helen; McElroy, C. Robert; Abou-Shehada, Sarah; Dunn, Peter J. (2015-12-21). "CHEM21 selection guide of classical- and less classical-solvents". Green Chem. 18 (1): 288–296. doi:10.1039/c5gc01008j. ISSN 1463-9270.
- ^ Diaz, Arthur F.; Drogos, Donna L. (2001-11-06). Oxygenates in Gasoline. ACS Symposium Series. Vol. 799. American Chemical Society. pp. 138–152. doi:10.1021/bk-2002-0799.ch010. ISBN 978-0841237605.
- ^ a b PubChem. "tert-Amyl methyl ether". pubchem.ncbi.nlm.nih.gov. Retrieved 2023-10-22.
- ^ "INEOS Oligomers Products". INEOS. Archived from the original on 2017-11-07. Retrieved 2017-10-30.
- ^ White, Russell D.; Daughtrey, Wayne C.; Wells, Mike S. (December 1995). "Health effects of inhaled tertiary amyl methyl ether and ethyl tertiary butyl ether". Toxicology Letters. 82–83: 719–724. doi:10.1016/0378-4274(95)03590-7. PMID 8597132.