
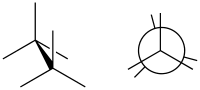
In organic chemistry, a staggered conformation is a chemical conformation of an ethane-like moiety abcX–Ydef in which the substituents a, b, and c are at the maximum distance from d, e, and f; this requires the torsion angles to be 60°.[1] It is the opposite of an eclipsed conformation, in which those substituents are as close to each other as possible.
Such a conformation exists in any open chain single chemical bond connecting two sp3-hybridised atoms, and is normally a conformational energy minimum. For some molecules such as those of n-butane, there can be special versions of staggered conformations called gauche and anti; see first Newman projection diagram in Conformational isomerism.
Staggered/eclipsed configurations also distinguish different crystalline structures of e.g. cubic/hexagonal boron nitride, and diamond/lonsdaleite.
YouTube Encyclopedic
-
1/3Views:99 79325 09376 655
-
Newman Projections - Anti, Gauche, Staggered, Eclipsed Energy Diagrams / Stability Organic Chemistry
-
Anti, Gauche, Staggered, Eclipse, andTotally Eclipse Conformations of Butane
-
Conformations of ethane | Organic chemistry | Khan Academy
Transcription
See also
References
- ^ Eliel, Ernest L.; Wilen, Samuel H. (1994). Stereochemistry of Organic Compounds. Wiley. p. 1207. ISBN 978-0-471-01670-0.
