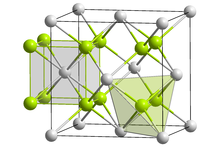
 Crystal structure of sodium polonide
__ Na+ __ Po2− | |
| Names | |
|---|---|
| Preferred IUPAC name
Sodium polonide | |
| Identifiers | |
3D model (JSmol)
|
|
| |
| |
| Properties | |
| Na2Po | |
| Molar mass | 254.96 g/mol |
| Appearance | greyish[1] |
| Related compounds | |
Other anions
|
|
Other cations
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Sodium polonide is a radioactive chemical compound with the formula Na2Po. This salt is a polonide, a set of very chemically stable compounds of polonium.[2][3] Due to the difference in electronegativity (ΔEN) between sodium and polonium (≈ 1.1 under the Pauling system) and the slight non-metallic character of polonium, it is intermediate between intermetallic phases and ionic compounds.
YouTube Encyclopedic
-
1/4Views:20 5841 3365 177371
-
Drawing Ionic Lewis Dot Structures (group 1 and 16)
-
Learn to write balanced chemical formulae. From www.flashscience.co.uk
-
Q1 Write down the formulae of:
-
GCSE ionic bonding L2: Ionic formulae
Transcription
Production
This salt may be produced from the reaction between aqueous polonium hydride and sodium metal:[2][3]
- H2Po + 2 Na → Na2Po + H2
This method of synthesis is hampered by the chemical instability of hydrogen polonide.
Sodium polonide may also be produced by heating sodium and polonium together at 300–400 °C.[1]
Crystal structure
Like lithium polonide and potassium polonide, sodium polonide has the antifluorite structure.[2][3]
References
- ^ a b Bagnall, K. W. (1962). "The Chemistry of Polonium". Advances in Inorganic Chemistry and Radiochemistry. New York: Academic Press. pp. 197–230. ISBN 9780120236046. Retrieved June 17, 2012.
- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 899. ISBN 978-0-08-022057-4.
- ^ a b c Moyer, Harvey V. (1956), "Chemical Properties of Polonium", in Moyer, Harvey V. (ed.), Polonium, Oak Ridge, Tenn.: United States Atomic Energy Commission, pp. 33–96, doi:10.2172/4367751, TID-5221.
