| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
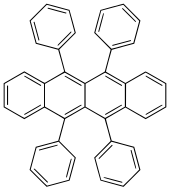
5,6,11,12-Tetraphenyltetracene | |
| Other names
5,6,11,12-Tetraphenylnaphthacene, rubrene
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.007.494 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C42H28 | |
| Molar mass | 532.7 g/mol |
| Melting point | 315 °C (599 °F; 588 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Rubrene (5,6,11,12-tetraphenyltetracene) is the organic compound with the formula (C18H8(C6H5)4. It is a red colored polycyclic aromatic hydrocarbon. Because of its distinctive optical and electrical properties, rubrene has been extensively studied. It has been used as a sensitiser in chemoluminescence and as a yellow light source in lightsticks.[1]
YouTube Encyclopedic
-
1/3Views:4 660 86135 8386 218
-
Make Glow Sticks - The Science
-
Vapor Activated Glow Stick
-
Raman Spectroscopy for Molecular Electronics
Transcription
Greetings fellow nerds. In this video we're going to make glow sticks of various colors and explain a few interesting points about them. But first I need to crush your expectations. So let's begin. First we start with the solvent diethyl phthalate. We can't use water because some of the chemicals don't work in water. Now we add some dye to give it color. I have here four different dyes but first we're going to start with 9,10-bis(phenylethynyl)anthracene. It looks orange in the solid state, but it dissolves to a green color. Let me shake it up. As you can see it is green when dissolved. This will give us a green color for the glow stick. Now we add the key ingredient bis(2,4,6-trichlorophenyl)oxalate, also known as TCPO. This stuff is what makes glow sticks work and provides just the right type of chemical energy to generate light when mixed with a fluorescent dye. Now I'm adding sodium acetate as a base. This reaction works better in alkaline conditions than in acidic conditions. Sodium bicarbonate and sodium salicylate also work if you don't have sodium acetate on hand. Ok we're ready. The final ingredient is hydrogen peroxide. This reacts with the TCPO and decomposes it, generating the chemical energy that transfers to the dye and gives it the glow. Let me get the lights. Oh look at that, it's not fully mixed, but it's already starting to react. Let me give it a shake. There we go. Nice! And that is a green glow stick. Ok let's try a few more colors. Lights! Wait up for my camera to rebalance. Ok let's start with a new batch of diethyl phthalate. Our next dye is rubrene. It looks bright red in the solid state, but after mixing you can see here it gives a bright orange color and will give a bright yellow glow. Once again we add in our TCPO. Other chemicals that can be used include bis(2,4-dinitrophenyl)oxalate, also known as DNPO. And bis(2,4,5-trichlorophenyl-6-carbopentoxyphenyl)oxalate, also known as CPPO. A lot of peroxylates can be used, but the ones I mentioned are most useful. Now for the sodium acetate. In a real glow stick all the chemicals are mixed first, but the hydrogen peroxide is kept separate in its own glass tube. When you break the tube the peroxide mixes and starts the reaction. That's why glow sticks make that cracking sound, it's the glass tube breaking. Here we are just going to add it directly. Lights! Whoa, that is really bright! It's much brighter than the green one. Let me put them side-by-side. The green one isn't dead, just my camera has set the contrast auto-balance too low. See when I take away the yellow one the camera can readjust the contrast. I can tell you personally they are actually both very bright, but the yellow one is much, MUCH, brighter. Okay, let's set those aside and try another dye. First, the diethyl phthalate solvent. Now the dye we're going to use is 9,10-diphenylanthracene. It's an off-white color in the solid state, and dissolves to give a clear solution. Now all these dyes have to be fluorescent, non-fluorescent dyes do not work. It's the fluorescent color that the dye ultimately glows with. 9,10-diphenylanthracene is clear normally but will actually give us a blue light. Okay, now let me add the TCPO and the sodium acetate. Some of you might ask if Luminol can be used. Luminol is a very different substance and works by a different reaction. While it does glow, it is much weaker and does not last as long as TCPO and similar type chemicals. Anyway, here we are with the hydrogen peroxide. Lights! Now that is a nice blue color. It actually looks closer to violet or purplish, but the camera doesn't quite pick it up perfectly. Let me get the other ones. As you can see, the yellow one with the rubrene dye is still the brightest. Uhh... looks like the green one separated a little, ah well. A lot of people ask how long these last. And that depends on how much of the chemicals you use, and what temperature the solution is at. The amount I use here at room temperature can last several hours to a day or two. Okay, let's try our last dye. This is Rhodamine B. It's green in the solid state. But as you guessed by now. very few things I do are as they appear. When it dissolves it gives us this deep red color and that is also the color that it will glow. Okay, let's add our TCPO and sodium acetate. Now there is a viral video out there that says you can make Mountain Dew glow with just baking soda and hydrogen peroxide. I can give you my expert scientific opinion that it's fake as well as all the so-called successful copies. Mountain Dew does not have the right chemicals for it. But if you don't believe me, just try it yourself, experimentation is the core of the scientific method. Okay enough ranting, here we are with the hydrogen peroxide. Lights! This takes a little longer to activate than the others. And that is red. Let me get the other ones to compare. Now Rhodamine isn't actually used in glow sticks much because, as you can see, it's decaying slowly and will die out sooner than the rest. Anyway, that is how you make different colored glow sticks. Some of you will probably ask how a white glow stick is made; it's actually very simple. All you do is mix together yellow and blue. Oh that's a nice multicolor effect. Anyway let me give it a shake. And there you go, that is a white glow stick. I used a tiny bit too much yellow, but here is one where I got the mixture right, just so you get the idea. Now a lot of people ask what happens if you don't use any dye at all and if that will give you a white light anyway. The answer is no, let me show you. I'm going to mix up a batch without the dye. Now the reason why it doesn't work is because the chemicals don't actually release light, they release energy. This energy must then transfer into a fluorescent dye to generate light. This might sound odd but fluorescent dyes work a little differently than normal absorption based dyes. Normal absorption based dyes work by absorbing particular colors and then reflecting or passing through other colors. Now fluorescent dyes also do this but they can also generate light by converting light of high energy, like ultraviolet, into light of lower energy, like the visible colors you see. Normal dyes don't have this mode of operation. The TCPO and hydrogen peroxide chemicals generate chemical energy that a fluorescent dye can convert into visible light, without needing ultraviolet light. If they directly generated light then we could see it without the dye. Okay enough talk, the best of science is testing our theories. LIGHTS! I'm adding in the hydrogen peroxide now. And as you can see, nothing visible is happening. The chemical reaction is going though, but without a fluorescent dye to convert the energy it's just being lost as heat. Here's a comparison of the theories. I think it's pretty obvious which one as more supporting evidence. TCPO does NOT generate light, not even UV light. It generates energy, which must transfer into a fluorescent dye. And that's the basic science of glow sticks. Thanks for watching, please subscribe, rate and comment.
Electronic properties
As an organic semiconductor, the major application of rubrene is in organic light-emitting diodes (OLEDs) and organic field-effect transistors, which are the core elements of flexible displays. Single-crystal transistors can be prepared using crystalline rubrene, which is grown in a modified zone furnace on a temperature gradient. This technique, known as physical vapor transport, was introduced in 1998.[2][3]
Rubrene holds the distinction of being the organic semiconductor with the highest carrier mobility, reaching 40 cm2/(V·s) for holes. This value was measured in OFETs prepared by peeling a thin layer of single-crystalline rubrene and transferring to a Si/SiO2 substrate.[4]
Crystal structure
Several polymorphs of rubrene are known. Crystals grown from vapor in vacuum can be monoclinic,[5] triclinic,[6] and orthorhombic motifs.[7] Orthorhombic crystals (space group Bbam) are obtained in a closed system in a two-zone furnace at ambient pressure.[8]
Synthesis
Rubrene is prepared by treating 1,1,3-Triphenyl-2-propyn-1-ol with thionyl chloride.[9]
The resulting chloroallene undergoes dimerization and dehydrochlorination to give rubrene.[10]
Redox properties
Rubrene, like other polycyclic aromatic molecules, undergoes redox reactions in solution. It oxidizes and reduces reversibly at 0.95 V and −1.37 V, respectively vs SCE. When the cation and anion are co-generated in an electrochemical cell, they can combine with annihilation of their charges, but producing an excited rubrene molecule that emits at 540 nm. This phenomenon is called electrochemiluminescence.[11]
References
- ^ Sawatzki-Park, Michael; Wang, Shu-Jen; Kleemann, Hans; Leo, Karl (2023). "Highly Ordered Small Molecule Organic Semiconductor Thin-Films Enabling Complex, High-Performance Multi-Junction Devices". Chemical Reviews. 123 (13): 8232–8250. doi:10.1021/acs.chemrev.2c00844. PMC 10347425. PMID 37315945.
- ^ Laudise, R.A; Kloc, Ch; Simpkins, P.G; Siegrist, T (1998). "Physical vapor growth of organic semiconductors". Journal of Crystal Growth. 187 (3–4): 449. Bibcode:1998JCrGr.187..449L. doi:10.1016/S0022-0248(98)00034-7.
- ^ Jurchescu, Oana Diana (2006) "Low Temperature Crystal Structure of Rubrene Single Crystals Grown by Vapor Transport" in Molecular organic semiconductors for electronic devices, PhD thesis Rijksuniversiteit Groningen.
- ^ Hasegawa, Tatsuo and Takeya, Jun (2009). "Organic field-effect transistors using single crystals". Sci. Technol. Adv. Mater. 10 (2): 024314. Bibcode:2009STAdM..10b4314H. doi:10.1088/1468-6996/10/2/024314. PMC 5090444. PMID 27877287.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Taylor, W. H. (1936). "X-ray measurements on diflavylene, rubrene, and related compounds". Zeitschrift für Kristallographie. 93 (1–6): 151. doi:10.1524/zkri.1936.93.1.151. S2CID 101491070.
- ^ Akopyan, S. A.; Avoyan, R. L. and Struchkov, Yu. T. Z. Strukt. Khim. 3, 602 (1962)
- ^ Henn, D. E. & Williams, W. G. (1971). "Crystallographic data for an orthorhombic form of rubrene". J. Appl. Crystallogr. 4 (3): 256. doi:10.1107/S0021889871006812.
- ^ Bulgarovskaya, I.; Vozzhennikov, V.; Aleksandrov, S.; Belsky, V. (1983). Latv. PSR Zinat. Akad. Vestis, Fiz. Teh. Zinat. Ser. 4. 53: 115
- ^ Furniss, B. Vogel's Textbook of Practical Organic Chemistry (5th ed.). pp. 840–841.
- ^ Furniss, B. Vogel's Textbook of Practical Organic Chemistry (5th ed.). pp. 844–845.
- ^ Richter, M. M. (2004). "Electrochemiluminescence (ECL)". Chemical Reviews. 104 (6): 3003–36. doi:10.1021/cr020373d. PMID 15186186.


