| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
2H-Pyran, 4H-Pyran
| |||
| Other names
2H-Oxine, 4H-Oxine
| |||
| Identifiers | |||
3D model (JSmol)
|
| ||
| ChemSpider | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C5H6O | |||
| Molar mass | 82.102 g·mol−1 | ||
| Related compounds | |||
Related compounds
|
Dihydropyran Tetrahydropyran | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
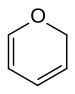
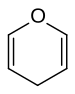
In chemistry, pyran, or oxine, is a six-membered heterocyclic, non-aromatic ring, consisting of five carbon atoms and one oxygen atom and containing two double bonds. The molecular formula is C5H6O. There are two isomers of pyran that differ by the location of the double bonds. In 2H-pyran, the saturated carbon is at position 2, whereas, in 4H-pyran, the saturated carbon is at position 4.
4H-Pyran was first isolated and characterized in 1962 via pyrolysis of 2-acetoxy-3,4-dihydro-2H-pyran.[1] It was found to be unstable, particularly in the presence of air. 4H-pyran easily disproportionates to the corresponding dihydropyran and the pyrylium ion, which is easily hydrolyzed in aqueous medium.
Although the pyrans themselves have little significance in chemistry, many of their derivatives are important biological molecules, such as the pyranoflavonoids.
The term pyran is also often applied to the saturated ring analog, which is more properly referred to as tetrahydropyran (oxane). In this context, the monosaccharides containing a six-membered ring system are known as pyranoses.
YouTube Encyclopedic
-
1/2Views:291 437708
-
Carbohydrates - cyclic structures and anomers | Chemical processes | MCAT | Khan Academy
-
3,4-dihydro-2H-pyran (DHP) preparation and protection of alcohol protection: complete mechanism.
Transcription
- Alrightie, so we've been speaking so far about carbohydrates as chains of carbon atoms. And these are chains of carbon atoms that feature an aldehyde or a ketone functional group. And that falls into this general kinda one-to-two-to-one ratio of carbon, hydrogen, and oxygen. And of course I'll keep using glucose as an example. Now I've also used the term polyhydroxylated to refer to the numerous hydroxyl groups that are in these carbohydrates. And really I bring all of this verbiage back up to hopefully spark your ability to see that carbohydrates have all the makings of an internal, or intramolecular, I guess, reaction between the carbonyl carbon here, and one of the hydroxyl groups. Because essentially what we have is carbonyl and alcohol chemical reactions kinda just waiting to happen. What happens when an alcohol nucleophile attacks an aldehyde or a ketone? Well, if there's an excess of alcohol, we end up with a product that is either an acetal, or a ketal. But what happens if there's only one nucleophilic attack by an alcohol, if we just have one alcohol? And that's gonna be the case in the ring-closing, intramolecular reaction we have going on here. Well in that case, we end up with a hemiacetal, or a hemiketal. And really that terminology is just kind of a review of acetal and ketal chemical reactions that would fall under, I guess if you're looking in an organic chemistry book, aldehyde or ketone reactions, probably in the carbonyl section. So let's show how this process is happening in the context of our glucose, over here. First, I'm gonna highlight the particular hydroxyl oxygen that's gonna act as the nucleophile. So, we'll make that pink. And after being deprotonated, so after losing this proton, this oxygen is gonna have an extra set of electrons right here. And those electrons are gonna target that carbonyl carbon. So I'll draw the carbonyl carbon in green. And remember that the carbonyl carbon has a partial positive charge on it. It has a partial positive charge, because a lot of the electron density in this double bond is being hogged by this oxygen, so the oxygen has a partially negative charge, and the carbonyl carbon is partially positive. And that makes it a perfect target for the nucleophile that's been created in the deprotonation process of this oxygen. And so after the oxygen's electrons attack this carbonyl carbon, what's gonna happen is the electrons from this double bond are kinda gonna kick back up to the oxygen up here, and eventually they're gonna attract another proton, and will form another hydroxyl group out of some of the electrons from that bond. Now you might be asking, and it's a perfectly valid question, why it's this particular oxygen, the one I highlighted, that's acting as the nucleophile. And you're gonna see as soon as I get the product drawn, that we've formed a six-member ring, so it really has to do with product stability. And if you remember, the basis for the formation of the ring in the first place, was the increased stability over the straight carbon chain. So it makes sense that we're gonna form the most stable ring that we can. Now when we end of with a six-membered carbohydrate ring, such as the case with glucose here, we call the product a pyranose. The -ose again, as the suffix for sugar. And the pyra- part, to indicate that this ring is a sugar with six carbons. And then if the carbohydrate ring is a five-carbon ring, we call it a furanose, which is a bit easier for me to remember, because furanose and five both start with the letter F. So that's kinda the memory jogger for me. And maybe a good example for that would be ribose, with its five-carbon chain, but I'll kinda stop there, because almost every ring-forming carbohydrate that I can think of with biological implications, at least, forms either a five- or a six-membered ring. So pyranoses and furanoses. So just by convention, you can see that I have placed the O in this corner up here, and that places the formerly carbonyl carbon down here right below it. And it's actually no longer the carbonyl carbon, but it's still significant because it's the only carbon here that is bonded to two oxygen atoms, the highlighted oxygen and it's bonded to another hydroxyl group, as well. So I'll keep a distinguishing color. And we also distinguish its name now as the anomeric carbon. So that's the anomeric carbon. And then we can go ahead and fill in the rest of the substituents in the diagram. So, a hydroxyl group and another, and another. And we call this diagram a Haworth diagram. So, Haworth diagram. And the Haworth diagram doesn't show us the actual configuration of the ring, because in reality, six-membered rings are gonna show up in a more-stable chair shape. But it is beneficial in telling us which substituents are above or below the ring. So to keep this convention straight in my mind, I remember the phrase, "Downright uplefting." So, "Downright uplefting." Kind of a play on, I guess, the phrase, "That's downright uplifting." But, "Downright uplefting," because as I fill in the substituents, those on the right side of the Fisher diagram will point down, and those on the left side of the Fischer diagram are gonna point up. So we can actually see that that one's up, and we'll make sure that this numbers off right. This one's up as well, And maybe we'll start numbering with one, two, three, four, five, six. And we can do that over here. This would be one, two, three, four, five, six. So our three carbon in the Haworth diagram is pointed up, and our three carbon on the Fischer diagram has its substituent on the left. So down, right, up, left. And as we get to the last carbon group, which kinda forms this tail down here, I remember that if it's a D-sugar, that group is gonna point up in the Haworth projection. So this is a D-sugar. And you can see in the Haworth projection that this last carbon points up as well. And really this is gonna be the case for a lot of sugar chemistry that you deal with, because again, we're entomatically programmed to digest D-sugars, so we often end up with this last group pointing up. Now the last thing I want to show you is the chair confirmation, so the chair confirmation. And that's because this is the kind of diagram that's gonna give us a sense for the actual configuration of D-glucose. But it really does just follow suit with the Haworth projection as far as the substituents being above or below the ring. So let me just kinda keep filling in the substituents here. I'll number them off, again, just so you can kind of see that there's some consistency here. So we've got one, two, three, four, five, six. And again this three-carbon right here is the only one with the hydroxyl group pointing up. And I guess I better change the color of our one-carbon, to keep that consistent, as well. Now I didn't indicate the position of the anomeric carbon's hydroxyl group yet, because I think it makes more sense to show it in this diagram. Remember that the original nucleophilic attack by the oxygen way back over here, that could've created two different products: one with an R configuration about the anomeric carbon, and the other with a S configuration. So that last hydroxyl group can actually be in two different positions. One one hand, the hydroxyl group would be cis to the last carbon in the equatorial position. So it'd be cis to this last carbon over here. And it's in a equatorial position. And we call this the beta anomer. Then on the other hand, I guess, it can be trans to that last carbon group, which would place it in the axial position down here. So I guess it could be down here in the axial position, and we call it the alpha anomer when the hydroxyl group is in the axial. And I kinda remember that a little bit easier: alpha for the axial position of that substituent. And I guess I've also heard that if fishies are down in the sea and birds are up in there air. So if that helps you keep them straight, you might be able to use that also. Now you gotta remember that what caused this ring to close in the first place, was some amount of acid or base. And the amount of acid and base in water is actually kinda capable of doing that. Because that's what facilitated this ring-closing process in the first place. And in water, the ring can actually open and close spontaneously. And when it opens up, the C1 and C2 bond right here can actually rotate, and when it closes again you can form either the alpha or the beta product. So this thing is constantly opening and closing to form the two different products. And we call that process, where it opens, and rotates, and closes again, mutarotation. So this thing is mutarotating in the water at all times. So mutarotation. And the outcome is that we end up with both configurations, the beta and the alpha, in the equilibrium concentration. So for glucose, that's gonna be about 36 percent alpha, and about 64 percent beta. And the reason that the alpha configuration is less favored in equilibrium for glucose, is because the transpositioning of the hydroxyl group creates some stearic hindrance. But this is pretty individualized for different sugars. So, I guess the most general rule, I suppose, that you could apply to all cyclic sugars, would be to say that the beta anomer, again anomer, that the beta anomer is the one with the anomeric oxygen, in the cis position with respect to the last carbon.
See also
References
- ^ Masamune, S.; Castellucci, N. T. (1962). "γ-Pyran". Journal of the American Chemical Society. 84 (12): 2452–2453. doi:10.1021/ja00871a037.