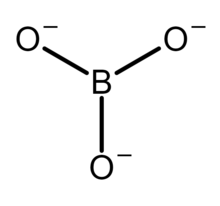
 Skeletal formula of the orthoborate anion
| |
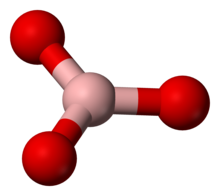 Ball-and-stick model of the orthoborate anion.
| |
| Names | |
|---|---|
| IUPAC name
Borate
| |
| Other names
trioxidoborate(3-)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| [BO3]3− | |
| Molar mass | 58.81 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
In inorganic chemistry, an orthoborate is a polyatomic anion with formula [BO3]3− or a salt containing the anion; such as trisodium orthoborate (Na+)3[BO3]3−. It is one of several boron oxyanions, or borates.
The name is also used in organic chemistry for the trivalent functional group B(O−)3, or any compound (ester) that contains it, such as triethyl orthoborate B(OC2H5)3.
Structure
The orthoborate ion is known in the solid state, for example, in calcium orthoborate (Ca2+)3([BO3]3−)2,[1] where it adopts a nearly trigonal planar structure. It is a structural analogue of the carbonate anion [CO3]2−, with which it is isoelectronic. Simple bonding theories point to the trigonal planar structure. In terms of valence bond theory, the bonds are formed by using sp2 hybrid orbitals on boron.
Some compounds termed orthoborates do not necessarily contain the trigonal planar ion. For example, gadolinium orthoborate GdBO3 contains the planar [BO3]3− ion only a high temperatures; otherwise it contains the polyborate anion [B3O9]9−.[2]
Reactions
Solution in water
When orthoborate salts are dissolved in water, the anion converts mostly to boric acid B(OH)3 and other hydrogen-containing borate anions, mainly tetrahydroxyborate [B(OH)4]−. The reactions of orthoborate in solution are therefore mostly those of these compounds.
In particular, these reactions include the condensation of tetrahydroxoborate with cis-vicinal diols such as mannitol, sorbitol, glucose and glycerol, to form relatively stable anion esters. This reaction is used in analytic chemistry to determine the concentration of borate anions.
See also
References
- ^ Vegas, A. (1985). "New description of the (Ca2+)3([BO3]3-)2 structure". Acta Crystallographica Section C. 41 (11): 1689–1690. doi:10.1107/S0108270185009052. ISSN 0108-2701.
- ^ Ren, M.; Lin, J. H.; Dong, Y.; Yang, L. Q.; Su, M. Z.; You, L. P. (1999). "Structure and Phase Transition of GbBO3". Chemistry of Materials. 11 (6): 1576–1580. doi:10.1021/cm990022o. ISSN 0897-4756.
