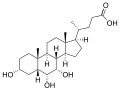
Muricholic acids are a group of bile acids found as one of the main forms in mice, which gives them their name, and at low concentrations in other species.[1] Muricholic acids differ from the primary bile acids found in humans, cholic acid and chenodeoxycholic acid, by having a hydroxyl group in the β-configuration at the 6-position. The orientation of the hydroxyl group at the 7-position defines α- or β-muricholic acid. Muricholic acids are detectable at low concentrations in human urine.[2]
The three major bile acids in germ-free mice are cholic acid, α-muricholic, and β-muricholic acids.[3] In conventional mice with a normal microbiome, ω-muricholic acid, and various sulfated forms are also found. Conjugation with taurine (to give tauromuricholic acids which are the main forms), or with glycine (to give glycomuricholic acids) takes place in the liver before secretion.[citation needed]
The enzyme responsible for the 6-hydroxylation reactions forming muricholates in rodents is the cytochrome P450 Cyp2c70. This produces α-muricholic acid from chenodeoxycholic acid, and β-muricholic acid from ursodeoxycholic acid.[4]
Tauromuricholic acids were shown to be potent antagonists of the bile acid receptor farnesoid X receptor (FXR).[5]
Chemical structures
-
α-muricholic acid
-
β-muricholic acid
-
γ-muricholic acid (hyocholic acid)
-
ω-muricholic acid
References
- ^ Russell DW (2003). "The enzymes, regulation, and genetics of bile acid synthesis". Annu. Rev. Biochem. 72: 137–74. doi:10.1146/annurev.biochem.72.121801.161712. PMID 12543708.
- ^ Goto, J; Hasegawa, K; Nambara, T; Iida, T (1992). "Studies on steroids. CCLIV. Gas chromatographic-mass spectrometric determination of 4- and 6-hydroxylated bile acids in human urine with negative ion chemical ionization detection". Journal of Chromatography. 574 (1): 1–7. doi:10.1016/0378-4347(92)80091-4. PMID 1629271.
- ^ Eyssen HJ, Parmentier GG, Mertens JA (July 1976). "Sulfate bile acids in germ-free and conventional mice". Eur. J. Biochem. 66 (3): 507–14. doi:10.1111/j.1432-1033.1976.tb10576.x. PMID 954753.
- ^ Takahashi, S; Fukami, T; Masuo, Y; Brocker, CN; Xie, C; Krausz, KW; Wolf, CR; Henderson, CJ; Gonzalez, FJ (December 2016). "Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans". Journal of Lipid Research. 57 (12): 2130–2137. doi:10.1194/jlr.M071183. PMC 5321228. PMID 27638959.
- ^ Sayin SI, Wahlström A, Felin J, et al. (February 2013). "Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist". Cell Metab. 17 (2): 225–35. doi:10.1016/j.cmet.2013.01.003. PMID 23395169.