| |
| Names | |
|---|---|
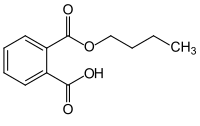
| Preferred IUPAC name
2-(Butoxycarbonyl)benzoic acid | |
| Other names | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.580 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H14O4 | |
| Molar mass | 222.240 g·mol−1 |
| Appearance | White solid |
| Melting point | 73.5 °C (164.3 °F; 346.6 K) |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H360 | |
| P201, P202, P281, P308+P313, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1,000 mg kg−1 (mouse, intraperitoneal)[1][2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Monobutyl phthalate (MBP) is an organic compound with the condensed structural formula CH3(CH2)3OOCC6H4COOH. It is a white solid that features both an butyl ester group and a carboxylic acid group. It is the major metabolite of dibutyl phthalate. Like many phthalates, MBP has attracted attention as a potential endocrine disruptor.[3]
MBP is also the secondary metabolite of butyl benzyl phthalate, less than monobenzyl phthalate (MBzP). It hydrolyses to phthalic acid and 1-butanol.[4]
YouTube Encyclopedic
-
1/1Views:407
-
Lecture 22, Chapter 4, Isothermal Reactor Design - Tutorial: PFR/PBR in series with separator
Transcription
References
- ^ a b c d e f "Monobutyl phthalate". PubChem. National Center for Biotechnology Information, U.S. National Library of Medicine. January 4, 2020. Retrieved January 10, 2020.
- ^ Chambon, Pierre; Riotte, Maurice; Daudon, Marc; Chambon-Mougenot, Renée; Bringuier, Janine (1971). "Etude du métabolisme des phtalates de dibutyle et de diéthyle chez le Rat" [Metabolism of dibutyl and diethyl phthalates in the rat]. Comptes Rendus de l'Académie des Sciences, Série D (in French). 273 (22): 2165–2168. PMID 5003086.
- ^ Hu Y, Dong C, Chen M, Chen Y, Gu A, Xia Y, Sun H, Li Z, Wang Y (2015). "Effects of monobutyl phthalate on steroidogenesis through steroidogenic acute regulatory protein regulated by transcription factors in mouse Leydig tumor cells". Journal of Endocrinological Investigation. 38 (8): 875–884. doi:10.1007/s40618-015-0279-6. PMID 25903692. S2CID 21965989.
- ^ Huang, Jingyu; Nkrumah, Philip N.; Li, Yi; Appiah-Sefah, Gloria (2013). "Chemical Behavior of Phthalates Under Abiotic Conditions in Landfills". Reviews of Environmental Contamination and Toxicology. Vol. 224. New York, NY: Springer Science+Business Media. pp. 39–52. doi:10.1007/978-1-4614-5882-1_2. ISBN 9781461458814. PMID 23232918.
