Molecular electronics is the study and application of molecular building blocks for the fabrication of electronic components. It is an interdisciplinary area that spans physics, chemistry, and materials science. The unifying feature is use of molecular building blocks to fabricate electronic components. Due to the prospect of size reduction in electronics offered by molecular-level control of properties, molecular electronics has generated much excitement. It provides a potential means to extend Moore's Law beyond the foreseen limits of small-scale conventional silicon integrated circuits.[1]
YouTube Encyclopedic
-
1/5Views:9 4609378788881 019
-
A Quantum of Solace - Molecular Electronics of Benzodiazepines (Google Workshop on Quantum Biology)
-
Chemical Sciences | D4S8 19/35 Molecular electronics and plasmonics: Electrons,... - Abraham Nitzan
-
Professor Paul Low – Bringing molecular electronics to the surface
-
Molecular Electronics (Mark Li the Science Guy)
-
Molecular electronics
Transcription
bjbjLULU >> TURIN: Let me first thank Hartmut Neven for the invitation at Google. This is--the whole field is very exciting. I think the way that Hartmut presented it was spot on. I would like to make two comments on his intro. One is, this is perhaps half frivolous but there's no absolute proof that the human brain does not factor large numbers, okay? And in fact, people like Ramanujan would be considered evidence to the contrary. So, what--I guess what I'm saying is that we know so little about how the brain works so we can't role out that it actually does things by brute force, okay? And the second thing is that I believe, and this addresses Hartmut's comment on Stuart Hameroff, I think that we're in the age of guesses here, we're guessing. People are putting their money on certain hypothesis. I personally think the brain is a quantum computer. If it can be done at all, it will have been done by the four billion a year R&D program. And I think Stuart was the first to basically state that guess, which is still a guess, but I think it's correct. I--and we're working on hunches here, but everybody, every scientist worth a damn has to have a hunch. And I think that hunch, which was Stuart's contribution, will turn out to be correct, okay? So let me see if events prove me wrong. Now... >> Actually, it was Penrose's idea about the quantum and eye of the micro tubule, but thank you for... >> TURIN: Okay, Penrose-Hameroff, sorry. Now, going back to [INDISTINCT] categories, we--he had level 1, trivial; level 2, semi-interesting; and level 3, interesting. My talk will be firmly stuck at 1.2, okay, just barely rising above the trivial. The thing is, not being a quantum mechanics specialist, I find even the trivial really interesting. You guys have been living such a high life with entanglement that you've become completely blas about the ordinary. But let me assure you there's plenty of ordinary quantum mechanics in biology that deserves worrying about. So, this is the first time I give this talk so bear with me if there are any sort of rough edges. But the work I'm going to talk about was done by myself in collaboration with two very good condensed metaphysicists Marshall Stoneham and Andrew Horsfield. And I'm a biophysicist, Marshall and Andrew are physicists, and we were dealing with a lot of chemistry so when we ran out of chemistry, which was several times, we asked Roald Hoffmann at Cornell, who very patiently, very patiently answered some seriously stupid questions. And Abdel Rawashdeh who did some computations with Roald Hoffmann, he was a post doc there. So benzodiazepines and I'm sorry I should mention that all the calculations I'd done were done on SCM's wonderful software suite ADF, which is a densely functional theory software which is suited to idiots like myself and the people at SCM are incredibly helpful. And, of course, DARPA provides the energy for all of this. That's my email in case anybody wants to get in touch. So, benzodiazepines. This is a picture of Leo Sternbach, who died two years ago, who discovered benzodiazepines accidentally in the late '50s. And he's holding in his hand, I haven't quite been able to figure out which one it is because I'm not sure what that green atom is, is it a bromine or a chlorine. But I suspect it's chlordiazepoxide, which was the very first one called Librium. And he was a wonderfully candid pharmaceutical chemist. It's very rare that you see chemist admitting to the fact that they don't, you know, they don't have a clue and they just made it--you know, they basically wanted to have some fun with chemistry and some of the products turned out to work. So, this statement of his, "Our knowledge of the processes occurring in the brain was rather limited," I think is the understatement of the century. So, their knowledge may have been limited and--but they got extremely lucky. And in the early '60s, they discovered a string of molecules including for example Lorazepam. Now I assume everybody in this room has taken benzodiazepines at least once. How many people here have never taken benzodiazepines? I don't believe that for a second. I don't believe that for a second, you probably have or you don't even know it. >> Generic terms, please. >> TURIN: Librium, Valium, Rohypnol if you're lucky, Temesta. >> Aspirin not a vote? Ambion? >> TURIN: Ambion, no. No, Ambion's different. Ambion's a non-benzodiazepine. Okay, let's have a vote again. >> Prozac. >> TURIN: Oh, I don't still don't believe it. Okay, you guys have far too clean a life. So benzodiazepines are extremely potent, remarkably potent molecules, they work at milligrams levels in the human body of, you know, 70 to 80 kilos. They're sedatives, they're hypnotics, they're axiolytics. The really powerful ones like Rohypnol for example, which we'll come back to in a second, are a mixture of all these things. They can send you to sleep. They can make you completely relaxed. They're used as pre-op medication for most, even minor operations. Some of them are--cause amnesia, which is a--Medazolam for example makes you forget what happened in the previous half hour which is--if there ever was a philosophical drug, that has to be it. You feel the pain when they have set your shoulder and then 10 minutes later you've forgotten that it happened. Did it happen? Nobody knows. So the terminology is that that's nitrobenzodiazepine, you add up own, you add a nice ring with the electrone withdrawing group on the side. You add another methyl and you end up with Flunitrazepam which is Rohypnol, also known as roofies. What's interesting about the Rohypnol molecule, and this is the first thing that Roald Hoffmann pointed out to me, was that there's a big delocalized--there's a big conjugated system of electron bonds here that goes all the way up to the imide. Imide is not a double bond thing but it's sufficiently conjugated. It behaves like a double bond in this kind of context. And so, here you have a molecule which has the ability to carry electrons from one end to the other quite easily. And you'll see that this is probably important. So where do benzodiazepines act? Well, if you Google it, which you--since this is the right place to mention Google, if you Google it you will find these lovely drawings done by different artists. They all act, the benzodiazepines act on the GABA receptor. So let me just single out one of these fellows. And this is a artist rendering of the GABA receptor in what I call squash notation, okay? In other words, all the subunits of the proteins are depicted as colorful volumes with some shading that look like the squashes you buy in supermarkets in this season. And squash notation in biology means you don't know how it works. Anytime you see that in a biology article, remember that's an admission of ignorance, okay? And what's really interesting about benzodiazepines is that they work on the GABA receptor together with a host of other things, in this particular instance you have up top left you have Medazolam, which is benzodiazepine Propofol, which I'm sure Stuart is very familiar with, which is a general anesthetic. Pregnenolone, which is another general anesthetic but a steroid general anesthetic. Ethanol, you're familiar with that. How many people have never--okay. Isoflurane and, of course, GABA. Isoflurane is the main, one of the main inhalational anesthetics. And so these are a remarkable class of compounds. The one I've highlighted on the right, Bretazenil, is probably not very well known to any of you. It's a molecule that's been proposed as an ethanol substitute by enterprising British pharmacologists who believed that we should stop drinking booze because it doesn't do the job very well and that we should actually actively develop an alternative to alcohol. This is a deeply unpopular notion but I think it has a great future. And I just wanted to mention this wonderful molecule, which is a promising candidate to replace--I haven't tried it. And of course, all this stuff does, does have its usefulness. Now, how do we know these things work on the GABA receptor? People say, you know, these drugs act on the GABA receptors. How do you find out that they do? Well, what you do is, depicted here. You inject the RNA for the different bits of the GABA receptors into a frog egg, an unfertilized frog egg. You wait for a couple of hours until it obligingly makes the receptors in its membrane. You put two electrodes, two micropipets, in the frog egg using one of these machines on the left, and you measure the current that goes to the membrane when you put on GABA, which is what the GABA receptor's sensitive to. And you get a trace, so the smaller of the two electrical traces is the current in response to GABA, and then you would put on some benzodiazepines and lo and behold the response has increased. The receptor is responding to GABA and is responding to GABA more strongly when there's benzodiazepines around. And this is the fundamental finding and it's--this is what I'm interested. I'm trying to figure out why the receptors cares about whether there are benzodiazepines around or not. So the GABA receptor is a membrane protein made of several subunits and it sits with up an exposed part at the top, which is exposed to the extracellular medium, and much of it is buried in the membrane. And it's made of five subunits; the alpha, then beta-alpha-beta, that makes four; and then a gamma subunit. These are different proteins and each of them comes in different flavors. So you can have alpha 1, alpha 2, alpha 3 so you can--sorting out the GABA receptor has been the job of microbiology and pharmacology for the last 20 years. It's a very complex receptor system. And the benzodiazepine binding site is between the alpha and the gamma. If there's no gamma, it doesn't respond. If there's the wrong kind of alpha, and we'll come back to that later, it doesn't respond. And that's a benzodiazepine on the same scale without the hydrogens being shown. So how do these things work? Well, the standard issue explanation that comes from pharmacology, in general, is that drugs work by a lock-and-key mechanism so but what is meant by that is actually non-trivial. What happens when you put a drug onto a receptor is that it binds to it. And what you see here is a molecule coming in and the receptor binding to the molecule by two different, say, interactions. At the top, you might have a hydrogen bond, and at the bottom, van der Waals interactions. And the theory of lock-and-key interactions is that the energy of binding is used to turn the receptor on, okay? That's where the energy is coming from, okay? Somebody has to turn the key and the energy for turning the key is the binding energy. So one of the things about--that's important about the lock-and-key idea is, of course, the potency of a molecule should somehow have something to do with its shape. If it's a key you've got to be turning, you know, it has to have the right shape. Unfortunately for us, or perhaps fortunately, the way that Leo Sternbach engineered it, so to speak, benzodiazepines, was to have fun as a chemist. And therefore, he put in as many modifiable positions on the benzodine because what he wanted to do was synthesize thousands of compounds, that's what chemists enjoy doing, and so you have all these positions on the benzodiazepine ring that have been explored. And what is remarkable is that not much sense, really, has come out of this vast pharmacology as to why one particular one works and another one doesn't. So let me just go through that. First of all, in order to illustrate structure-activity relations, I've used a thread on a Russian drug, do-it-yourself drug chemistry site, called Bluelight. Those guys are clearly really good, okay? Because this person says, "Anybody know how to make a better benzodiazepine," basically, as if you're going to do that in your kitchen, you know? And he gets the following answer, all right? And this is as good a summary. This is on a thread. This is as good a summary of structure-activity relations of benzodiazepines as you will find anywhere, and better than most, okay? So clearly, these people, somewhere in the middle of Russia, are worrying about making a better Rohypnol at home, okay? I find it--the Web has revealed all sorts of things. So, you can do all manner of stuff to the basic benzodiazepines skeleton and end up with useful drugs. All the things on the periphery here are useful, important drugs. And you can even try--this is a very unfortunate photograph of Corwin Hampsch, it makes him look like he's wanted by the police in several states. He's a very distinguished chemist who has been working for years on quantitative structure-activity relations. In other words, harnessing the power of statistics to try to understand from a vast database what are the important features. And he tackled the so-called QSAR, quantitative structure-activity relations, of benzodiazepines. And after 22 pages of dense statistics, what comes out is this, which is almost exactly nothing, okay? In other words, even Corwin Hampsch can't crack it and so--this is a British expression, an elephant gives birth to a mouse. So in order--to make things worse, just when Corwin Hampsch thought he was almost home, several new drugs have come out that have--that act on the same binding site and have nothing to do with benzodiazepine's structure, and they are listed--the three from left here--and they're called the Z drugs; Zolpidem, Zaleplon, Zopiclone, and that's Ambien, Sonata, and I forget the other one. You can see that the picture is getting bleaker and bleaker in terms of understanding how these things actually work. Now, a few years back there was--Villar, he was a--he's a quantum chemist, made an interesting observation, which was that there was a correlation between activity of benzodiazepines and the energy of the lowest unoccupied molecular orbital. And this, I remember reading this and thinking, "This is kind of weird and interesting." And then I forgot about it promptly for many years, until--okay, let me explain what--why I thought that was interesting. The reason why the energy of the LUMO may be important is if you're interested in charge-transfer, okay? If you have a charge-transfer--this is--these are two molecules that are used in chemistry as the exemplar of charge-transfer. TTF is extremely electron-rich and TCNQ is extremely electron-poor, okay? So you mix them together and they form a sandwich which--in which the electron basically gets transferred from the HOMO of TTF to the LUMO of TCNQ, which happened to be at roughly the same energy, and this thing conducts, okay? The electron--this is actually a room temperature metallic conductor. The solution looks--has a metallic luster, as far as I know. So, I thought this was, you know, when I saw the Villar's idea about benzodiazepine, I thought it was interesting. But it was interesting for me in a slightly different context, which is to do with whether proteins are conductors or not. One of the fundamental tenets of biochemistry is that proteins are not electronic conductors, okay? They're insulators, okay? And you can make them conduct but only under certain conditions, and these conditions were explored by a great man, Albert Szent-Gy rgyi. I assume some of you are familiar with him. He discovered vitamin C, got a Nobel Prize in '38, I think, for it, and then went on to do all manner of strange things, and he had his own lab in Woods Hole, and he found a millionaire that funded his research the rest of his life. Anybody here who wants to do that, there's my email in this thing. He didn't believe in writing grants because he said, "If I knew what I was going to do, I wouldn't be a scientist." Okay, so what he did was he studied the properties of protein-modified, chemically modified, to make the electrons move. And how he did it was this. He took an ordinary protein, casein, albumin, it doesn't matter, and you treat it with a compound called methylglyoxal, which is also called pyruvic aldehyde, for those who study biochemistry, and which is very closely related to the butter flavor in popcorn, it smells intensely buttery. And this thing is very reactive and it binds to the terminal amino groups of one particular amino acid, lysine. And when you--when it does that, it makes an imine. You will recall that the benzodiazepine ring has an imine in it so we're homing in onto the real problem here. So when you do that, you find--this is what Szent-Gy rgyi discovered--you find the proteins go black. They treat it with methylglyoxal, albumin, which is initially white, goes a dark brown. And that, to a physicist, is immediately a sign of electron mobility. What's happening is the electrons are absorbing--the electron transitions are absorbing light in the visible range, so you've lowered the energy from the ultraviolet, which is where it normally lies, to the visible. And you can see these are spectra. Spectrum three is native albumin, two, we'll forget about for the moment, and one is treated with methylgloxal. And this thing at the bottom is the wavelength. You see in the visible range, it absorbs pretty nicely, which is why it's brown. Now, Szent-Gy rgyi, of course, had he, in his day, had DFT software would have cracked everything and would have left nothing to us, but he didn't so you can calculate things that he couldn't calculate. And one of the things he didn't calculate is the HOMO-LUMO gap, the excitation gap, for untreated alpha helix with a lysine, and that's on the left and that's five electron volts, that's way in the UV. And you put an imine on it, you treat it with methylglyoxal and you look at the energy and there's three electron volts so it has come down quite a bit. And if you look back at that graph, the DFT predicts pretty accurately the peak of the absorption at 3.61 eV, which is in the violet. Now, those observant among you will notice that that excitation--that the absorption, sorry, of the protein here stretches all the way east of that, so that even though we correctly predict the 3.6, clearly, there are things that are enabling the protein to absorb at much lower energies. And for a long time, I think that was completely unclear as to what could be going on. Why would it absorb all the way into 450, say. And Szent-Gy rgyi's point of view, which is quoted in this line, was that electron mobility was a fundamental thing, okay? Now, no one these days would dream of say--of using the word "clumsy" to describe macromolecules. That's, in biology, that's sacrilege, okay? And arguably, it is sacrilege because macromolecules do extraordinary things, but he felt there was something missing. Now, the people's reaction to his work was, "Well, that's fine. But you've modified the methylglyoxal, that doesn't really happen, so who cares," okay? One word on that, methylglyoxal now is considered one of the primary agents of protein aging so that when an animal ages you get all manner of covalent reactions that happen between let's say sugars and other reactive species in the cell and one of them is methylglyoxal, okay? So I just want to put this in the back of your head that actually Szent-Gy rgi may have been looking at a physiological or pathological phenomenon rather than an artificial. But I think no one was interested because it seems too artificial. Now, what happened recently, when I say recently, I mean the last 20 years, when you get to my age recently extends further back. Peter Kovacic was in his early 80s and still very active, took the whole idea of imines one step further by implicating iminiums. The imine group can be proteinated and become an iminium and Peter Kovacic has documented the effect of iminium containing molecules in pharmacology and biochemistry all over the place. Now why is this interesting? Well, because you can do this and you can go from imine to iminium, you just proteinate it at a charge. Now what happens to the energies? We've seen that the unmodified thing huge, huge gap, no absorption is visible. The modified imine 3.6, let's say, but the iminium is 0.2 electron volts. Now 0.2 electron volts, now we're talking. This is no longer the UV or the visible; this is in darkness, okay? So if you managed to put an iminium somewhere, you may have electronic transitions from, let's say, the valance band to the conduction band so to speak, that happened in darkness. So you--what you've got yourself is a proper semiconductor. Okay, now, Kovacic pointed out that this could be possible but he--I don't think he's very fond of quantum chemistry software so I had to it myself. If you look at the electron affinity of benzodiazepines, you have the bare skeleton, the nitro group introduction makes--gives it electron affinity because nitro is electron withdrawing, you add another fluorine, you get a bit more electron affinity, but if you put that extra one hydrogen atom on it, all of a sudden the LUMO falls through the floor and you end up with an electron affinity with respect to vacuum of 5.4 eVs. Now that--the 5 eVs is the magic number because if--in order to take an electron from the vacuum into the molecule you gain 5 eVs that's basically valence band for solutes. How do we know that? Because it takes 5 eVs to get electrons out of the sun, that's the photoelectric effect. So this is not just theory, there's plenty of evidence out there that benzodiazepines actually do this in real life. In fact, they're the--just about the only drug that can be measured in an electron capture detector, directly without modification to the molecule. They have such a high electron affinity and you see them in electron capture detention. So, why is this interesting from a pharmacology standpoint? Well, because if you go back to the structure activity relation, the one that's never mentioned is the fact that if you don't have the imine, the C double bond N on the left, if you saturate that, okay, the diazepines don't work. That is a fundamental SAR. So you have to have the imine and therefore possibly the iminium in order for it to work. There are two exceptions, I know these days that people will look things up and then just email me so let me forced all that. There are two exceptions. There are two perfectly good benzodiazepines, don't start out with an imine; ketazolam and cloxazolam. They're pro-drugs that actually breakdown to the compound that has the imine before they start acting. Now, why is iminium really interesting? Well, it's interesting because all the drugs, all the sonata, the zolpidem, the zaleplon, zopiclone, they all have an iminium with PKs in the right regions. So this is beginning to look like it might actually do a bit more explaining. So can iminium cause charge-transfer and therefore conduction in dark in proteins? The Szent-Gy rgi charge-transfer complex was covalent. The TTF TCNQ is non-covalent. So we're thinking of a non-covalent, we're thinking of basically the drug sticking to the receptor and causing electrons to jump from protein to the drug. You can actually calculate that, I mean, it's boring. You can watch these calculations roll for 12 hours. I do that all the time; I just sit in front of my computer and watch the iterations. But you can calculate an alpha helix, there's a structure on the right. There's an alpha helix with two phenylalanines and you attach it to Rohypnol molecule and you calculate the HOMO-LUMO distance with and without the iminium. And without the iminium, just the imine, 2.8 electron volts, with the iminium, 0.19; more than a factor of 10, okay? So, and this--what this tells you is that if benzodiazepine formed iminiums, they're in the position to suck up electrons from proteins in such a way that the protein that gives the electron is probably going to conduct in the manner that Szent-Gy rgi discovered. Now how do we know that this may be happening in real life? Well, you have to know where to look is the answer because there's no direct measurements of connectivity of GABA receptors. Nobody is doing that, partly because nobody cares, but they've done it accidentally and this is what I'm going to talk about. People discovered some years back that you could alter GABA receptors' function by putting more of--by putting electrons, so to speak, in the outside medium, electron donors, they're called redox reagents, that if you changed the redox state of a receptor it would behave differently. I just want to say, by the way, that this discovery was made in Russia, in the then Soviet Union in the late '70s by Pupi and Kozachenko that--their paper by Pupi and Kozachenko was utterly, utterly ignored for 20 years, that they got zero credit for discovering this very important and interesting part of pharmacology and they're still getting very little credit, they're mentioned--they're sometimes cited and sometimes not. But this is a perfect illustration of how much good biophysics was going on in the eastern bloc that has just basically been forgotten. So what they do is they put drugs that push--redox reagents act on a particular type of chemical bond in proteins, the disulfide group, and if you push--you can push them towards making disulfide bridges between two amino acids or you can push them towards breaking the disulfide bridges. And what they find is totally remarkable. You're measuring the GABA receptor and you put GABA and it responds every time nicely. Then you put something that breaks disulfide bridges and you get a bigger response, that's the DTT in the middle, the peak of the red bit. And when you remove the DTT, it goes back. And that's perfectly normal because disulfide bridges will spontaneously--the two SHs will spontaneously reform to make a disulfide bridge so you'd expect the DTT to be reversible. But then another fun happens. You put DTNB which causes them to make a disulfide bridge and you'll find that the effect of DTNB is reversible, okay? Now this is very strange. This is just not an isolated observation. The other group who did that and published it the same time found exactly the same thing, almost the same trace, okay? So, why is this weird? Well, because the disulfide bridge they're working on is in the outside medium. It's actually known which one it is. There's only one, okay? And it's in this--in a place called the cys-loop of the subunits. So, there are no electron donors in the outside medium. Every disulfide should be as a bridge, should not be as two SHs. So the fact that you can--they should be fully oxidized, but when you put an external oxidant like DTNB, it acts transiently the thing clearly re-breaks. Something is keeping those extracellular sulfides reduced, okay? That something can only be electrons, there's nothing else that will work, and therefore the electrons must be coming from inside the cell. Now, I've contact--when I figured this out I contacted Trevor Smart, who had actually done the experiments, and he said "You know, we always wondered why these things were switching back but we couldn't figure it out." The biologists aren t used to thinking of electrons crossing membranes. So, let's just take a one--a step back here and look at how this circuit might work. You've got--the benzodiazepine binding site is on the outside between two subunits. You have the cys-loop which is not far from the benzodiazepine binding. And in fact, it's extremely close. It's only a fragment--it's along one of the protein ribbons. And where are the electrons coming from, okay? They're coming from inside. But they can't just--you can't just give an electron to a protein out of the--you know, in a random way, there has to be a binding site on the protein to accept electrons from an electron donor. So what I did was I went looking for electron, for sockets, so to speak, in the primary sequences of GABA receptors. And this G, this is a amino acid sequence, G is glycine, then anything than either glycine or alanine then anything, anything and then either glycine or alanine. So this GxGxxG or A is a diagnostic sequence for electron binding sites. Incidentally, there's nothing super-duper mysterious about this, these are very small amino acids, the smallest site chance. So basically, what you're doing is peeling off the insulator to expose the peptide backbone, okay? The peptide backbone is probably what's conducting so when you have glycines and alanines in this arrangement the donor molecule can approach the backbone enough to give electrons to it, okay? And you find this binding site in the alpha subunit, okay, of the type of subunits that are sensitive to benzodiazepine. So there's a very good indication that the socket is present in the right place and the socket binds a soluble electron donor. So here is the electron circuit that I'm postulating exists in the GABA receptor. You have NADH given--giving electrons to one sub-unit. The electrons travel across to the benzodiazepine binding site, jump across probably through the benzodiazepine, very likely, and reduce a cys-loop which then opens up and controls the function of the receptor. So what we're looking at here is actually not a lock-and-key. It's a field effect--a chemical field effect transistor. It's chemical because you need to proteinate the benzodiazepine in order to make it work, okay? And it's--once it's turned on, it acts as a gate for electron flow between subunits. Now, how would you test this? Well, you could--the thing about molecular biology is you can have fun, you can do all matter of stuff. You can mutate the NADH binding site, the electron socket. You put a big fat amino acid, it won't work anymore. Easy to do. I mean, on a scale of one to ten, three easy to do, okay, not ten. You can measure electron flow through the protein during the experiment. You can do--if you have electrons flowing, hopping through, you should see a change in capacitance, basically. The protein becomes conducting. It's in an insulator. You should see that as capacitance changing. You could try ligands with a very, very high electron affinity. In fact, I'm thinking some of the anesthetics have a very high electron affinity. It would be very interesting to see if you can trigger-off--if that's the mechanism that they used to turn on the GABA receptor. And finally, you can mutate the electron path to the--so, in conclusion, next time you take--well, those of you who don't take benzodiazepines will be unaffected by this, but next time, you take a breakfast of champions like this. When you're looking at these molecules in the form of pills, think of them as electronic components. You may be swallowing a bunch of transistors. This clearly--you would agree with me that this 1.1, right, triviality level? >> No. >> TURIN: It's--this is step one. I'm very concerned with doing things that are experimentally measurable because I'm not enough of a theoretician to have a good time with theory, okay? So, this is step one. But consider the possibility that there are actually quantum effects within the molecule. People who ve demonstrated wonderfully interesting--I mean since the beginning of molecular electronics, there's been an awful lot of talk about interference between different conducting paths of a molecule. I would--I, for one, would not be so surprised if somebody based on this tomorrow showed me that the reason some benzodiazepines work and some don't is because some have a constructive interference of electron flow through them and some have a destructive interference. This is very much step one. We don't know where this takes us. We don't know--I don't know whether it connects in any way with what Stuart is saying. But what I'm saying is, here's a situation where we thought we had it pretty much logged down and no electrons, no quantum stuff was involved, and it turns out it may be that the very--the level one quantum stuff is. Thank you very much. Yeah? >> I have a couple of questions. The first one is, this transition from imine to iminium. >> TURIN: Yes. >> Will this depend on pH of the sodium receptors? >> TURIN: Yes, absolutely. It depends on pH in that--in a completely normal way the PKs of these things can be anywhere between, let's say, two and eight. Of course, pH, bulk pH, is relatively meaningless if your drug is bound next to an acid group, you're effectively dealing with a very high local concentration of hydrogen ions. So, yes, it is pH dependent. But whether it's pH dependent in situ is not known. >> So, the second question is, you used in the sequence of GxGA[INDISTINCT]. >> TURIN: Yeah. >> Is this some kind of a universal statement or... >> TURIN: Yes. Let me just find the slide that deals with that. No. Yeah, that's the one. Yes, the GxGxxG is a universal--not universal, but is a very common binding sequence. >> Okay. >> TURIN: Stuart? >> Yeah. Thank you very much, Luca. Just to be clear, are you saying that the conduction is through non-polar [INDISTINCT] regions because I saw phenylalanine... >> TURING: Okay. >> ...complicated rings so these resident ring structures that they were trying to going through? >> TURIN: Well, not really. If the conduction has to take place over the entire span of the protein, it won't find, if you want, phenylalanine-type network to do so. Very likely, what's happening is conduction along the peptide backbone. And the reason for that is that the peptide bond itself is, of course, conjugated. But there's also--there are some interesting aspects, too, a sort of a hyper-conjugation such that the--it maybe that there's a low conductivity all the way along the backbone. This is too pretty contentious. But as DFT techniques improve--excuse me--and as in particular, as the linear scaling DFT that enables you to calculate whole proteins comes on-stream, we'll find out whether the electronic structure proteins actually includes low-lying conduction band states directly. >> How far apart would your mad rings have to be to conduct going to phenylalanine? >> TURIN: I think Elizabeth can probably answer this one better than I. I think the answer is it depends how fast. The tunneling phenomenon, of course, scales viciously with distance, okay, exponential distance squared. So, if you're only interested in one electron every two weeks, they can probably be quite far, okay? The thing about these receptors is that, as you saw, the time scale of reduction is tens of milliseconds, possibly. So, and two electrons every hundred milliseconds is not, you know, is not a very stringent requirement for tunneling, that's a low tunneling current. So the answer is probably farther than we think right now. >> We were talking a little bit about this during the break, but how does the drug molecule find its way there? Does diffusion explain everything? >> TURIN: Yeah. I think--I don't think this--I mean I think diffusion explains--let me clarify this, diffusion explains how it finds its way to the active site. Shape explains why it binds to the active site with high affinity, okay? But binding isn't everything. You can have perfectly well have an antagonist. What we call antagonists in pharmacology are things that bind like mad and do nothing, okay? And in fact, the flumazenil, which is the number one antagonist for benzodiazepine does not have any imide on it. It just has the right shape, okay? So, the equation--the mentals that are sort of association of affinity with efficiency is, of course, very easy to make. Pharmacologists don't make that mistake. They have another thing which is called efficacy, okay? So something can bind like crazy and do precisely nothing because it has an efficacy of zero. The problem is, once you've called it efficacy, you haven't--still haven't got a clue as to what is actually happened, okay? Thank you. >> Does [INDISTINCT] occur in other types of drug interaction, other than psychoactive drugs? >> TURIN: Other than psychoactive drugs? >> Yeah. >> TURIN: Yes. If you read Kovacic's papers, he has found iminiums in all matter of strange places. I can't recall all the categories, but the answer is yes. Yes, absolutely. Thank you very much. gd64 gd64 :p64 urn:schemas-microsoft-com:office:smarttags City urn:schemas-microsoft-com:office:smarttags country-region urn:schemas-microsoft-com:office:smarttags place >> TURIN: Let me first thank Hartmut Neven for the invitation at Google Owner Normal.dot CJ Anand Microsoft Office Word >> TURIN: Let me first thank Hartmut Neven for the invitation at Google Title Microsoft Office Word Document MSWordDoc Word.Document.8
Molecular scale electronics
| Part of a series of articles on |
| Nanoelectronics |
|---|
| Single-molecule electronics |
| Solid-state nanoelectronics |
| Related approaches |
| Portals |
|
|
Molecular scale electronics, also called single-molecule electronics, is a branch of nanotechnology that uses single molecules, or nanoscale collections of single molecules, as electronic components. Because single molecules constitute the smallest stable structures possible, this miniaturization is the ultimate goal for shrinking electrical circuits.
Conventional electronic devices are traditionally made from bulk materials. Bulk methods have inherent limits, and are growing increasingly demanding and costly. Thus, the idea was born that the components could instead be built up atom by atom in a chemistry lab (bottom up) as opposed to carving them out of bulk material (top down). In single-molecule electronics, the bulk material is replaced by single molecules. That is, instead of creating structures by removing or applying material after a pattern scaffold, the atoms are put together in a chemistry lab. The molecules used have properties that resemble traditional electronic components such as a wire, transistor, or rectifier. This concept of using a molecule as a traditional electronic component was first presented by Aviram and Ratner in 1974, when they proposed a theoretical molecular rectifier composed of donor and acceptor sites which are insulated from one another.[2]
Single-molecule electronics is an emerging field, and entire electronic circuits consisting exclusively of molecular sized compounds are still very far from being realized. However, the continuous demand for more computing power, together with the inherent limits of the present day lithographic methods make the transition seem unavoidable. Currently, the focus is on discovering molecules with interesting properties and on finding ways to obtain reliable and reproducible contacts between the molecular components and the bulk material of the electrodes.
Molecular electronics operates at distances less than 100 nanometers. Miniaturization down to single molecules brings the scale down to a regime where quantum mechanics effects are important. In contrast to the case in conventional electronic components, where electrons can be filled in or drawn out more or less like a continuous flow of electric charge, the transfer of a single electron alters the system significantly. The significant amount of energy due to charging has to be taken into account when making calculations about the electronic properties of the setup and is highly sensitive to distances to conducting surfaces nearby.

One of the biggest problems with measuring on single molecules is to establish reproducible electrical contact with only one molecule and doing so without shortcutting the electrodes. Because the current photolithographic technology is unable to produce electrode gaps small enough to contact both ends of the molecules tested (in the order of nanometers) alternative strategies are put into use. These include molecular-sized gaps called break junctions, in which a thin electrode is stretched until it breaks. One of the way to over come the gap size issue is by trapping molecular functionalized nanoparticles (internanoparticle spacing is match able to the size of molecules) and later target molecule by place exchange reaction.[3] Another method is to use the tip of a scanning tunneling microscope (STM) to contact molecules adhered at the other end to a metal substrate.[4] Another popular way to anchor molecules to the electrodes is to make use of sulfur's high chemical affinity to gold; though useful, the anchoring is non-specific and thus anchors the molecules randomly to all gold surfaces, and the contact resistance is highly dependent on the precise atomic geometry around the site of anchoring and thereby inherently compromises the reproducibility of the connection. To circumvent the latter issue, experiments have shown that fullerenes could be a good candidate for use instead of sulfur because of the large conjugated π-system that can electrically contact many more atoms at once than a single atom of sulfur.[5] The shift from metal electrodes to semiconductor electrodes allows for more tailored properties and thus for more interesting applications. There are some concepts for contacting organic molecules using semiconductor-only electrodes, for example by using indium arsenide nanowires with an embedded segment of the wider bandgap material indium phosphide used as an electronic barrier to be bridged by molecules.[6]
One of the biggest hindrances for single-molecule electronics to be commercially exploited is the lack of means to connect a molecular sized circuit to bulk electrodes in a way that gives reproducible results. Also problematic is that some measurements on single molecules are done at cryogenic temperatures, near absolute zero, which is very energy consuming.
History
The first time in history molecular electronics are mentioned was in 1956 by the German physicist Arthur Von Hippel,[7] who suggested a bottom-up procedure of developing electronics from atoms and molecules rather than using prefabricated materials, an idea he named molecular engineering. However the first breakthrough in the field is considered by many the article by Aviram and Ratner in 1974.[8] In this article named Molecular Rectifiers, they presented a theoretical calculation of transport through a modified charge-transfer molecule with donor acceptor groups that would allow transport only in one direction, essentially like a semiconductor diode. This was a breakthrough that inspired many years of research in the field of molecular electronics.
Molecular materials for electronics
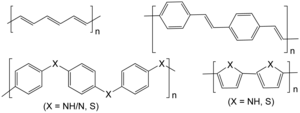
The biggest advantage of conductive polymers is their processability, mainly by dispersion. Conductive polymers are not plastics, i.e., they are not thermoformable, yet they are organic polymers, like (insulating) polymers. They can offer high electrical conductivity but have different mechanical properties than other commercially used polymers. The electrical properties can be fine-tuned using the methods of organic synthesis[9] and of advanced dispersion.[10]
The linear-backbone polymers such as polyacetylene, polypyrrole, and polyaniline are the main classes of conductive polymers. Poly(3-alkylthiophenes) are the archetypical materials for solar cells and transistors.[9]
Conducting polymers have backbones of contiguous sp2 hybridized carbon centers. One valence electron on each center resides in a pz orbital, which is orthogonal to the other three sigma-bonds. The electrons in these delocalized orbitals have high mobility when the material is doped by oxidation, which removes some of these delocalized electrons. Thus the conjugated p-orbitals form a one-dimensional electronic band, and the electrons within this band become mobile when it is emptied partly. Despite intensive research, the relationship between morphology, chain structure, and conductivity is poorly understood yet.[11]
Due to their poor processability, conductive polymers have few large-scale applications. They have some promise in antistatic materials[9] and have been built into commercial displays and batteries, but have had limits due to the production costs, material inconsistencies, toxicity, poor solubility in solvents, and inability to directly melt process. Nevertheless, conducting polymers are rapidly gaining attraction in new uses with increasingly processable materials with better electrical and physical properties and lower costs. With the availability of stable and reproducible dispersions, poly(3,4-ethylenedioxythiophene) (PEDOT) and polyaniline have gained some large-scale applications. While PEDOT is mainly used in antistatic applications and as a transparent conductive layer in the form of PEDOT and polystyrene sulfonic acid (PSS, mixed form: PEDOT:PSS) dispersions, polyaniline is widely used to make printed circuit boards, in the final finish, to protect copper from corrosion and preventing its solderability.[10] Newer nanostructured forms of conducting polymers provide fresh impetus to this field, with their higher surface area and better dispersability.
Recently supramolecular chemistry has been introduced to the field, which provide new opportunity for developing next generation of molecular electronics.[12][13] For example, two orders of magnitude current intensity enhancement was achieved by inserting cationic molecules into the cavity of pillar[5]arene.[14]
See also
- Comparison of software for molecular mechanics modeling
- Molecular conductance
- Molecular wires
- Organic semiconductor
- Single-molecule magnet
- Spin transition
- Unimolecular rectifier
- Nanoelectronics
- Molecular scale electronics
- Mark Ratner
- Mark Reed (physicist)
- James Tour
- Supramolecular chemistry
- Supramolecular electronics
References
- ^ Petty, M.C.; Bryce, M.R. & Bloor, D. (1995). Introduction to Molecular Electronics. New York: Oxford University Press. pp. 1–25. ISBN 0-19-521156-1.
- ^ Aviram, Arieh; Ratner, Mark A. (15 November 1974). "Molecular rectifiers". Chemical Physics Letters. 29 (2): 277–283. Bibcode:1974CPL....29..277A. doi:10.1016/0009-2614(74)85031-1.
- ^ Jafri, S H M; Blom, T; Leifer, K; Strømme, M; Löfås, H; Grigoriev, A; Ahuja, R; Welch, K (29 October 2010). "Assessment of a nanoparticle bridge platform for molecular electronics measurements". Nanotechnology. 21 (43): 435204. Bibcode:2010Nanot..21Q5204J. doi:10.1088/0957-4484/21/43/435204. PMID 20890018. S2CID 29398313.
- ^ Gimzewski, J.K.; Joachim, C. (1999). "Nanoscale science of single molecules using local probes". Science. 283 (5408): 1683–1688. Bibcode:1999Sci...283.1683G. doi:10.1126/science.283.5408.1683. PMID 10073926.
- ^ Sørensen, J.K. Archived 2016-03-29 at the Wayback Machine. (2006). "Synthesis of new components, functionalized with (60)fullerene, for molecular electronics". 4th Annual meeting - CONT 2006, University of Copenhagen.
- ^ Schukfeh, Muhammed Ihab; Storm, Kristian; Mahmoud, Ahmad; Søndergaard, Roar R.; Szwajca, Anna; Hansen, Allan; Hinze, Peter; Weimann, Thomas; Fahlvik Svensson, Sofia; Bora, Achyut; Dick, Kimberly A.; Thelander, Claes; Krebs, Frederik C.; Lugli, Paolo; Samuelson, Lars; Tornow, Marc (2013). "Conductance Enhancement of InAs/InP Heterostructure Nanowires by Surface Functionalization with Oligo(phenylene vinylene)s". ACS Nano. 7 (5): 4111–4118. doi:10.1021/nn400380g. PMID 23631558.
- ^ Von Hippel, Arthur R.; Landshoff, Rolf (October 1959). "Molecular Science and Molecular Engineering". Physics Today. 12 (10): 48. Bibcode:1959PhT....12j..48V. doi:10.1063/1.3060522.
- ^ Aviram, Arieh; Ratner, Mark A. (November 1974). "Molecular rectifiers". Chemical Physics Letters. 29 (2): 277–283. Bibcode:1974CPL....29..277A. doi:10.1016/0009-2614(74)85031-1.
- ^ a b c Naarmann, Herbert (2000). "Polymers, Electrically Conducting". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a21_429. ISBN 978-3-527-30673-2.
- ^ a b Wessling, B. (2000). "Conductive polymers as organic nanometals". Handbook of Nanostructured Materials and Nanotechnology. Vol. 5. pp. 501–575. doi:10.1016/B978-012513760-7/50062-9. ISBN 978-0-12-513760-7.
- ^ Skotheim, T., Elsenbaumer, R., Reynolds, J., Eds.; Handbook of Conducting Polymers, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 1998[page needed]
- ^ Chen, Hongliang; Fraser Stoddart, J. (September 2021). "From molecular to supramolecular electronics". Nature Reviews Materials. 6 (9): 804–828. Bibcode:2021NatRM...6..804C. doi:10.1038/s41578-021-00302-2. S2CID 232766622.
- ^ Yao, Yifan; Zhang, Lei; Orgiu, Emanuele; Samorì, Paolo (June 2019). "Unconventional Nanofabrication for Supramolecular Electronics" (PDF). Advanced Materials. 31 (23): 1900599. Bibcode:2019AdM....3100599Y. doi:10.1002/adma.201900599. PMID 30941813. S2CID 205290060.
- ^ Li, Xiaobing; Zhou, Siyuan; Zhao, Qi; Chen, Yi; Qi, Pan; Zhang, Yongkang; Wang, Lu; Guo, Cunlan; Chen, Shigui (21 February 2023). "Supramolecular Enhancement of Charge Transport through Pillar[5]arene-Based Self-Assembled Monolayers". Angewandte Chemie International Edition. 62 (19): e202216987. doi:10.1002/anie.202216987. PMID 36728903. S2CID 256502098.
Further reading
- Heath, James R. (1 August 2009). "Molecular Electronics". Annual Review of Materials Research. 39 (1): 1–23. Bibcode:2009AnRMS..39....1H. doi:10.1146/annurev-matsci-082908-145401.
External links
 Media related to Molecular electronics at Wikimedia Commons
Media related to Molecular electronics at Wikimedia Commons
