
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
(3R)-3,5-Dihydroxy-3-methylpentanoic acid | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C6H12O4 | |||
| Molar mass | 148.158 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
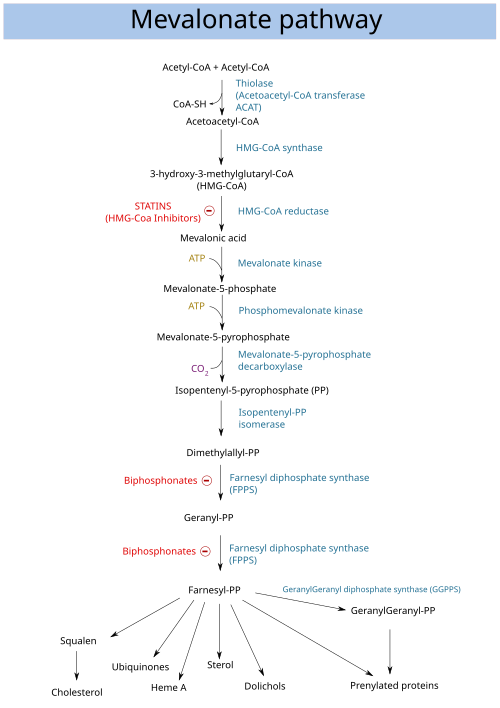
Mevalonic acid (MVA) is a key organic compound in biochemistry; the name is a contraction of dihydroxymethylvalerolactone. The carboxylate anion of mevalonic acid, which is the predominant form in biological environments, is known as mevalonate and is of major pharmaceutical importance. Drugs like statins (which lower levels of cholesterol) stop the production of mevalonate by inhibiting HMG-CoA reductase.[1]
YouTube Encyclopedic
-
1/3Views:24 3202 89340 389
-
Cholesterol Synthesis (Part 2 of 6) - Stage 1: Mevalonate Synthesis
-
From terpenes to steroids part 1 terpenes
-
Cholesterol Synthesis (Part 1 of 6) - Intro
Transcription
Chemistry
Mevalonic acid is very soluble in water and polar organic solvents. It exists in equilibrium with its lactone form, called mevalonolactone, that is formed by internal condensation of its terminal alcohol and carboxylic acid functional groups. Mevalonolactone acts to correct statin linked myopathy and limb girdle muscular disease caused by HMG co-A reductase mutation.[2]
Biology
Mevalonic acid is a precursor in the biosynthetic pathway known as the mevalonate pathway that produces terpenes and steroids. Mevalonic acid is the primary precursor of isopentenyl pyrophosphate (IPP), that is in turn the basis for all terpenoids. Mevalonic acid is chiral and the (3R)-enantiomer is the only one that is biologically active.
References
- ^ Endo, A. (1992). "The discovery and development of HMG-CoA reductase inhibitors". Journal of Lipid Research. 33 (11): 1569–1582. PMID 1464741.
- ^ Yogev Y, Shorer Z, Koifman A, Wormser O, Drabkin M, Halperin D, Dolgin V, Proskorovski-Ohayon R, Hadar N, Davidov G, Nudelman H, Zarivach R, Shelef I, Perez Y, Birk OS (February 2023). "Limb girdle muscular disease caused by HMGCR mutation and statin myopathy treatable with mevalonolactone". Proc Natl Acad Sci U S A. 120 (7): e2217831120. doi:10.1073/pnas.2217831120. PMC 9963716. PMID 36745799.


