This is a list of local anesthetic agents. Not all of these drugs are still used in clinical practice and in research. Some are primarily of historical interest.
| Drug | Other common names | Image | First synthesis | Dates of clinical use | Chemical/structural class | Duration of effect | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| amylocaine | Stovaine | 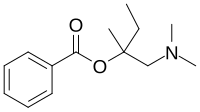
|
1904 (Ernest Fourneau) | ester- benzoic
|
||||||
| ambucaine |  |
diester - aminosalicylic | ||||||||
| articaine | Astracaine, Septanest, Septocaine, Ultracaine, Zorcaine | 
|
Amide | |||||||
| benzocaine | Anbesol, Orajel | 
|
Ester - Aminobenzoic | Short | ||||||
| benzonatate | Tessalon | 
|
||||||||
| bupivacaine | Marcaine, Sensorcaine, Vivacaine | 
|
1957 (Ekenstam) | 1963 (Widman and Telivuo) | Amide | Moderate | ||||
| butacaine | 
|
ester- aminobenzoic | ||||||||
| butanilicaine | 
|
Amide | ||||||||
| chloroprocaine | Nesacaine | 
|
Ester - Aminobenzoic | |||||||
| cinchocaine (INN) | dibucaine (USAN), Cincain, Cinchocaine, Nupercainal, Nupercaine, Sovcaine | 
|
1925 (Meischer) | 1930 (Uhlmann) | Ester - Aminobenzoic | |||||
| cocaine | 
|
1855 (first isolation by Friedrich Gaedcke), 1898 (first synthesis by Richard Willstätter) | 1884 (Karl Koller, William Stewart Halsted) | Ester - Benzoic | ||||||
| cyclomethycaine |  |
Ester - hydroxybenzoic | ||||||||
| dibucaine |  |
Amide | ||||||||
| diperodon |  |
|||||||||
| dimethocaine | larocaine |  |
||||||||
| eucaine | α-Eucaine, β-eucaine |
|
1900. α[6] β[7][8] | |||||||
| etidocaine | Duranest | 1971 (Takman) | 1972 (Lund) | |||||||
| hexylcaine | Cyclaine, Osmocaine | |||||||||
| fomocaine |  |
ester - phenyl | ||||||||
| fotocaine |  |
|||||||||
| hydroxyprocaine |  |
ester - aminosalicylic | ||||||||
| isobucaine |  |
Ester - benzoic | ||||||||
| levobupivacaine | Chirocaine | 1990s (Mather and Tucker) | 1995 | |||||||
| lidocaine[12][13]
(lignocaine) |
Xylocaine | 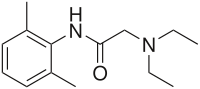
|
1943 (Nils Löfgren and Bengt Lundqvist) | 1947 (Torsten Gordh) | ||||||
| mepivacaine | Carbocaine, Polocaine | 1956 (Ekenstam and Egner) | 1957 (Dhuner) | |||||||
| meprylcaine | Epirocain | |||||||||
| metabutoxycaine | ||||||||||
| nitracaine | Ester- Aminobenzoic | |||||||||
| orthocaine | ||||||||||
| oxetacaine (oxethazaine) | ||||||||||
| oxybuprocaine | benoxinate, Novesine | |||||||||
| Paraethoxycaine |  |
|||||||||
| phenacaine | Holocaine | |||||||||
| piperocaine | metycaine | |||||||||
| piridocaine |  |
|||||||||
| pramocaine | pramoxine | |||||||||
| prilocaine | Citanest | 1959 (Nils Löfgren and Egner) | 1960 (Wielding) | |||||||
| Primacaine | ||||||||||
| procaine | Novocain, borocaine (procaine borate), ethocaine | 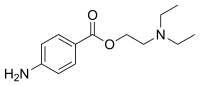
|
1904 (Alfred Einhorn) | 1905 (Heinrich Braun) | ||||||
| procainamide |  |
|||||||||
| proparacaine | proxymetacaine | |||||||||
| propoxycaine[16] | ||||||||||
| Pyrrocaine |  |
|||||||||
| quinisocaine (INN) | dimethisoquin (USAN) |  |
||||||||
| ropivacaine | Naropin | 1957 (Ekenstam) | 1997 | |||||||
| trimecaine | Mesdicain, Mesocain, Mesokain | |||||||||
| tetracaine | amethocaine, Dicaine, Pontocaine | 
|
1928 (O. Eisleb) | 1931 | ||||||
| Tolycaine |  |
|||||||||
| Tropacocaine[20] | 
|
YouTube Encyclopedic
-
1/3Views:185 524683 2451 005
-
Pharmacology - ANTIDEPRESSANTS - SSRIs, SNRIs, TCAs, MAOIs, Lithium ( MADE EASY)
-
3 People Who Probably Saved Your Life
-
Exposure to Early Psychosocial Deprivation Can Undermine Healthy Brain Development
Transcription
See also
- 4-Aminobenzoic acid
- Amino amide
- Amino esters
- Anesthesia
- Anesthetic
- Brachial plexus block
- Cocaine analogues: local anesthetics
- Dental anesthesia
- Dibucaine number
- Epidural
- Intravenous regional anesthesia
- Local anesthesia
- Local anesthetic with vasoconstrictor
- Local anesthetic toxicity
- Methemoglobin
- Sodium channel blocker
- Spinal anesthesia
- Topical anesthesia
- Veterinary anesthesia
References
- ^ Büchi, J; Stünzi, E; Flury, M; Hirt, R; Labhart, P; Ragaz, L (1951). "Über lokalanästhetisch wirksame basische Ester und Amide verschiedener Alkoxy-amino-benzoesäuren". Helvetica Chimica Acta. 34 (4): 1002–1013. doi:10.1002/hlca.19510340404.
- ^ S. M. McElvain and T. P. Carney, J. Amer. Chem. Soc, 68, 2592 (1946).
- ^ K. Miescher, Helv. Chim. Acta, 15, 163 (1932).
- ^ 44. T. H. Rider, J. Amer. Chem. Soc, 52, 2115 (1930).
- ^ M. S. Raasch and W. R. Brode, J. Amer. Chem. Soc, 64, 1112 (1942).
- ^ Harries, C. (1903). "Ueber ein neues p-Menthadiën aus Dihydrocarvylamin". Justus Liebig's Annalen der Chemie. 328 (3): 322–326. doi:10.1002/jlac.19033280303.
- ^ G. Meiling, Chem. Ber., 6, 173 (1896).
- ^ H. King, J. Chem. Soc, 125, 41 (1924).
- ^ a b Schoenberger, Matthias; Damijonaitis, Arunas; Zhang, Zinan; Nagel, Daniel; Trauner, Dirk (2014). "Development of a New Photochromic Ion Channel Blocker via Azologization of Fomocaine". ACS Chemical Neuroscience. 5 (7): 514–518. doi:10.1021/cn500070w. ISSN 1948-7193. PMC 4102962. PMID 24856540.
- ^ W. Grimme and H. Schmitz, Chem. Ber., 84, 734 (1917).
- ^ J. R. Reasenberg and S. D. Goldberg, J. Amer. Chem. Soc, 67, 933 (1945).
- ^ "Lignocaine - C14H22N2O". ChemSpider. Retrieved 2015-10-13.
- ^ "Lidocaine". The Merck Index Online. Retrieved 2015-10-13.
- ^ W. G. Christiansen and S. E. Harris, U. S. Patent 2,404,691 (1946).
- ^ L. A. Walter and R. J. Fosbinder, J. Amer. Chem. Soc, 61, 1713 (1939).
- ^ Clinton, R. O.; Salvador, U. J.; Laskowski, S. C.; Wilson, Mary (February 1952). "Derivatives of 4-Amino-2-hydroxybenzoic Acid. II". Journal of the American Chemical Society. 74 (3): 592–598. doi:10.1021/ja01123a005.
- ^ N. Lofgren, C. Tegner, and B. Takman, Acta Chem. Scand., 11, 1724 (1957).
- ^ T. H. Rider, J. Amer. Chem. Soc, 52, 2115 (1930).
- ^ R. Hiltmann, F. Mietzsch, and W. Wirth, U.S. patent 2,921,077 (1960).
- ^ Jowett, Hooper Albert Dickinson; Pyman, Frank Lee (1909). "CXVI.—Relation between chemical constitution and physiological action in the tropeines. Part II". J. Chem. Soc., Trans. 95: 1020–1032. doi:10.1039/CT9099501020.


