
In chemistry, Ferric refers to the element iron in its +3 oxidation state. Ferric chloride is an alternative name for iron(III) chloride (FeCl3). The adjective ferrous is used instead for iron(II) salts, containing the cation Fe2+. The word ferric is derived from the Latin word ferrum, meaning "iron".
Although often abbreviated as Fe3+, that naked ion does not exist except under extreme conditions. Iron(III) centres are found in many compounds and coordination complexes, where Fe(III) is bonded to several ligands. A molecular ferric complex is the anion ferrioxalate, [Fe(C2O4)3]3−, with three bidentate oxalate ions surrounding the Fe core. Relative to lower oxidation states, ferric is less common in organoiron chemistry, but the ferrocenium cation [Fe(C2H5)2]+ is well known.
YouTube Encyclopedic
-
1/3Views:57 9457 6721 491
-
Recover Copper from Ferric Chloride Etchant (Waste free method)
-
How to Mix Powdered Ferric Chloride Etchant for Copper, Brass or Nickel Etching
-
EL PLUGIN DE LA SEMANA: FERRIC TDS (Saturador)
Transcription
WARNING: This experiment handles boiling hydrochloric acid and sulfuric acid as well as toxic quantities of ionic copper and iron. Gloves should be worn and the experiment should be performed outside or in a fume hood. Greetings fellow nerds. In this video we’re going to recover the copper in a spent solution of ferric chloride printed circuit board etchant. As well as restore the ferric chloride etchant to working chemistry. And as an added challenge we’re going to do so without producing any additional chemical waste. So here is our spent ferric chloride etchant contaminated with copper chloride. Take note of the original volume as we’ll use that as a guide for restoring it later. I have about 400mL. Now filter the solution to remove any insoluble particles like bits of circuit board dust. You can wash down the filter paper with some deionized or distilled water if you want to maximize recovery. Now I’m getting the filtrate and distilling off the water and hydrochloric acid. This step is actually optional but i’m doing it because I wanted to weigh how much ferric and copper chlorides i had. You don’t actually need to do this part and can go right to adding copper. So this is what the raw mixture of spent ferric chloride and copper chloride looks like. Black crud. Now we add copper metal. We want to completely react all the remaining ferric chloride and copper chlorides. Now if you distilled off the acid to dryness like I did and weighed the residue then ratio to use is 3 to 1. For every 3 grams of spent ferric chloride etchant add in 1 gram of copper metal. This is a rather huge excess but we’re doing it to ensure the reaction goes to completion. If you didn’t distill and don’t know the mass of the ferric chloride then that’s okay, just make a guess and add a lot of copper. You can always go back and add more if there isn’t enough. I recommend using larger pieces of copper like i am here as it will make it easier to remove the copper later. Now since i distilled out the acid i’m going to add the acid back in. Okay, now we distill the mixture again. But this time the added copper will react with the ferric chloride and copper chlorides to produce ferrous chloride and cuprous chloride. These are also known as iron (II) chloride and copper (I) chloride. We’re doing this because while iron (II) chloride is soluble in water, copper (I) chloride is not, and that gives us our means to separate the two. Keep going with the distillation until it’s almost dry. It’s hard to see from this angle but the solution is considerably lighter as the reaction goes to completion. Finally the residue should be this light color of iron (II) chloride and copper (I) chloride rather than the black brown color of the iron (III) chloride and copper (II) chlorides. If it’s not, you need to let it cool, add the acid back in, and boil again until it is. You also need to make sure there is an excess of copper metal left over. If all the copper metal reacted then there is likely still unreacted Iron (III) chloride and copper (II) chlorides. So you’ll need to add in more copper metal when repeating the distillation. Anyway. Looks like my run worked out. Turn off the heating and let it cool. You can see here the cake of light green iron (II) chloride and white copper (I) chloride. Now take the residue and at least three times its mass of water. If you don’t know the mass then don’t worry, just add enough water to equal the original starting volume. The cake is fairly solid so you’re going to have to shake it a lot to break it up. We’re trying to dissolve the green Iron (II) chloride and separate it from the insoluble copper (I) chloride. At the same time try and remove any copper metal pieces if you can. You might have to pour it out and back in several times to do so. As you shake it up, you’ll might notice a rusty color develop on the sides of the glassware. What’s happening is the air is actually oxidizing the iron (II) chloride into iron oxide, which is rust. While it certainly looks like horrible contamination, the amount is actually very tiny. So don’t worry too much about if it’s happening. Okay now once the solid residue is dissolved and it’s just a suspension of fine white particles, filter the solution. It is at this point that we are separating the copper from the iron. You can also take this opportunity to remove any remaining bits of copper metal. Be sure to wash out any remaining residues with water to ensure maximum recovery. Once everything is filtered, retrieve the residue of copper (I) chloride. And there you have it, separation of the iron from the copper. Now we need to convert the copper chloride back into the copper metal that went into it. Now it just so happens i already have a video on that goes into that process in full detail so I won’t waste your time here. You can go right to the weighing part and proceed to convert the copper chloride to copper sulfate, distill off the hydrochloric acid, and electrolyze the copper sulfate to recover the copper. The process is no different. Now we deal with the iron (II) chloride. Boil down to a volume that corresponds to the difference between how much hydrochloric acid was recovered and the original volume. Since i recovered 300 mL of hydrochloric acid, and my original volume of ferric chloride was 400 mL, i’ll need to boil down to 100 mL. My solution a bit darker than originally because I performed this part of the video a few days after the filtration. In that time air had oxidized some of the iron (II) chloride into iron (III) chloride and some iron hydroxides. This is not a problem. Anyway, once you have boiled off the appropriate amount of water, let it cool and then add the hydrochloric acid back in. Now this solution still contains mostly iron (II) chloride as well as hydrochloric acid. To convert it to iron (III) chloride, or ferric chloride. Get an aquarium pump and bubble air through it until it becomes the characteristic dark yellow of ferric chloride. What’s happening is the oxygen in the air is reacting with the iron (II) chloride and hydrochloric acid to produce iron (III) chloride, also known as ferric chloride. And there you have it. We have now recovered the copper from spent ferric chloride PCB etchant and regenerated the etchant for reuse. And we’ve done so without producing additional chemical waste. Thanks for watching.
Iron(III) in biology
All known forms of life require iron, which usually exists in Fe(II) or Fe(III) oxidation states.[1] Many proteins in living beings contain iron(III) centers. Examples of such metalloproteins include oxyhemoglobin, ferredoxin, and the cytochromes. Many organisms, from bacteria to humans, store iron as microscopic crystals (3 to 8 nm in diameter) of iron(III) oxide hydroxide, inside a shell of the protein ferritin, from which it can be recovered as needed. [2]
Insufficient iron in the human diet causes anemia. Animals and humans can obtain the necessary iron from foods that contain it in assimilable form, such as meat. Other organisms must obtain their iron from the environment. However, iron tends to form highly insoluble iron(III) oxides/hydroxides in aerobic (oxygenated) environment, especially in calcareous soils. Bacteria and grasses can thrive in such environments by secreting compounds called siderophores that form soluble complexes with iron(III), that can be reabsorbed into the cell. (The other plants instead encourage the growth around their roots of certain bacteria that reduce iron(III) to the more soluble iron(II).)[3]
The insolubility of iron(III) compounds is also responsible for the low levels of iron in seawater, which is often the limiting factor for the growth of the microscopic plants (phytoplankton) that are the basis of the marine food web.[4]
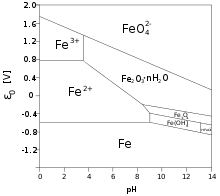
Iron(III) salts and complexes
Typically iron(III) salts, like the "chloride" are aquo complexes with the formulas [Fe(H2O)5Cl]2+, [Fe(H2O)4Cl2]+, and [Fe(H2O)3Cl3]. Iron(III) nitrate dissolved in water to give [Fe(H2O)6]3+ ions. In these complexes, the protons are acidic. Eventually these solutions hydrolyze producing iron(III) hydroxide Fe(OH)3 that further converts to polymeric oxide-hydroxide via the process called olation. These hydroxides precipitates out of the solution as solids. That reaction liberates hydrogen ions H+ lowering the pH of its solutions. The equilibria are elaborate:[5]
- [Fe(H2O)6]3+ ⇌ [Fe(H2O)5OH]2+ + H+
- [Fe(H2O)5OH]2+ ⇌ [Fe(H2O)4(OH)2]+ + H+
- 2 [Fe(H2O)4(OH)2]+ ⇌ [Fe2(H2O)8(OH)2]+2 + 2 H2O
Various chelating compounds prevent the polymerization. These same ligands can even dissolve iron(III) oxides and hydroxides. One of these ligands is EDTA, which is often used to dissolve iron deposits or added to fertilizers to make iron in the soil available (soluble) to plants. Citrate also solubilizes ferric ion at neutral pH, although its complexes are less stable than those of EDTA. Many chelating ligands - the siderophores - are produced naturally to dissolve iron(III) oxides.
The aquo ligands on iron(III) complexes are labile. This behavior is visualized by the color change brought about by reaction with thiocyanate:
- [Fe(H2O)6]3+ + SCN− ⇌ [Fe(SCN)(H2O)5]2+ + H2O
Whereas [Fe(H2O)6]3+ is nearly colorless, [Fe(SCN)(H2O)5]2+ is deep red.
While iron(III) aquo complexes tend to convert to polymeric oxy-hydroxides, iron(III) complexes with other ligands form stable solutions. The complex with 1,10-phenanthrolinebipyridine is soluble and can sustain reduction to it iron(II) derivative:

Iron(III) minerals and other solids

Iron(III) is found in many minerals and solids, e.g., oxide Fe2O3 (hematite) and iron(III) oxide-hydroxide FeO(OH) are extremely insoluble reflecting their polymeric structure. Rust is a mixture of iron(III) oxide and oxide-hydroxide that usually forms when iron metal is exposed to humid air. Unlike the passivating oxide layers that are formed by other metals, like chromium and aluminum, rust flakes off, because it is bulkier than the metal that formed it. Therefore, unprotected iron objects will in time be completely turned into rust.
Bonding

Iron(III) is a d5 center, meaning that the metal has five "valence" electrons in the 3d orbital shell. The number and type of ligands bound to iron(III) determine how these electrons arrange themselves. With so-called "strong field ligands" such as cyanide, the five electrons pair up as best they can. Thus ferricyanide ([Fe(CN)6]3− has only one unpaired electron. It is low-spin. With so-called "weak field ligands" such as water, the five electrons are unpaired. Thus aquo complex ([Fe(H2O)6]3+ has only five unpaired electrons. It is high-spin. With chloride, iron(III) forms tetrahedral complexes, e.g. ([Fe(Cl)4]−. Tetrahedral complexes are high spin. The magnetism of ferric complexes can show when they are high or low spin.
See also
- Ferric chloride – Inorganic compound (Iron(III) chloride)
- Ferric oxide – Chemical compound (Iron(III) oxide)
- Ferric fluoride – chemical compound (Iron(III) fluoride)
- Ferrous – The element iron in its +2 oxidation state
References
- ^ "Iron integral to the development of life on Earth – and the possibility of life on other planets". University of Oxford. 7 December 2021. Retrieved 9 May 2022.
- ^ Berg, Jeremy Mark; Lippard, Stephen J. (1994). Principles of bioinorganic chemistry. Sausalito, Calif: University Science Books. ISBN 0-935702-73-3.
- ^ H. Marschner and V. Römheld (1994): "Strategies of plants for acquisition of iron". Plant and Soil, volume 165, issue 2, pages 261–274. doi:10.1007/BF00008069
- ^ Boyd PW, Watson AJ, Law CS, et al. (October 2000). "A mesoscale phytoplankton bloom in the polar Southern Ocean stimulated by iron fertilization". Nature. 407 (6805): 695–702. Bibcode:2000Natur.407..695B. doi:10.1038/35037500. PMID 11048709. S2CID 4368261.
- ^ Earnshaw, A.; Greenwood, N. N. (1997). Chemistry of the elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0-7506-3365-4.
