| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
1H-Indol-3-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.216.308 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
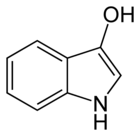
| C8H7NO | |
| Molar mass | 133.14728 |
| Hazards | |
| GHS labelling: | |
 
| |
| Danger | |
| H302, H311, H319, H400 | |
| P264, P270, P273, P280, P301+P312, P302+P352, P305+P351+P338, P312, P322, P330, P337+P313, P361, P363, P391, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
In organic chemistry, indoxyl is a nitrogenous substance with the chemical formula: C8H7NO.[1][2] Indoxyl is isomeric with oxindol and is obtained as an oily liquid.
Indoxyl is obtained from indican, which is a glycoside. The hydrolysis of indican yields β-D-glucose and indoxyl.
Indigo dye is a product of the reaction of indoxyl with a mild oxidizing agent such as atmospheric oxygen.
Indoxyl can be found in urine and is titrated with Obermayer's reagent, which is a dilute solution of ferric chloride (FeCl3) in hydrochloric acid (HCl).[3]
YouTube Encyclopedic
-
1/3Views:5 31069 6811 171
-
Introduction to Chemical Biology 128. Lecture 08. RNA.
-
Blue white screening of DNA clones
-
Herbs of India: Ayurvedic and Tibetan knowledge of wild herbs. With English subtitles
Transcription
References
- ^ Katritzky, A. R.; Pozharskii, A. F. (2000). Handbook of Heterocyclic Chemistry (2nd ed.). Academic Press. ISBN 0080429882.
- ^ Clayden, J.; Greeves, N.; Warren, S.; Wothers, P. (2001). Organic Chemistry. Oxford, Oxfordshire: Oxford University Press. ISBN 0-19-850346-6.
- ^ Lide, David (1998). CRC - Handbook of Chemistry and Physics. CRC press LLC. pp. Section 8 page 3. ISBN 0-8493-0479-2.
