| |||
| Names | |||
|---|---|---|---|
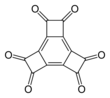
| Preferred IUPAC name
Tetracyclo[8.2.0.02,5.06,9]dodeca-1,5,9-triene-3,4,7,8,11,12-hexone | |||
| Other names
Hexaoxotricyclobutabenzene
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C12O6 | |||
| Molar mass | 240.12 g mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Hexaoxotricyclobutabenzene is an organic compound with formula C12O6. It can be viewed as the sixfold ketone of tricyclobutabenzene.
It is an oxide of carbon, detected by 13C NMR in 2006.[1][2]
References
- ^ Hamura, T.; Ibusuki, Y.; Uekusa, H.; Matsumoto, T.; Siegel, J. S.; Baldridge, K. K.; Suzuki, K. (2006). "Dodecamethoxy- and Hexaoxotricyclobutabenzene: Synthesis and Characterization". Journal of the American Chemical Society. 128 (31): 10032–10033. doi:10.1021/ja064063e. PMID 16881630.
- ^ Butenschön, H. (2007). "A new oxocarbon C12O6 via highly strained benzyne intermediates". Angewandte Chemie. 46 (22): 4012–4014. doi:10.1002/anie.200700926. PMID 17508349.