 | |
| Clinical data | |
|---|---|
| Trade names | GS-6620 |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
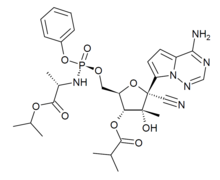
| Formula | C29H37N6O9P |
| Molar mass | 644.622 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
GS-6620 is an antiviral drug which is a nucleotide analogue. It was developed for the treatment of Hepatitis C but while it showed potent antiviral effects in early testing,[1][2] it could not be successfully formulated into an oral dosage form due to low and variable absorption in the intestines which made blood levels unpredictable.[3][4] It has however continued to be researched as a potential treatment for other viral diseases such as Ebola virus disease.[5][6]
References
- ^ Cho A, Zhang L, Xu J, Lee R, Butler T, Metobo S, et al. (March 2014). "Discovery of the first C-nucleoside HCV polymerase inhibitor (GS-6620) with demonstrated antiviral response in HCV infected patients". Journal of Medicinal Chemistry. 57 (5): 1812–25. doi:10.1021/jm400201a. PMID 23547794.
- ^ Feng JY, Cheng G, Perry J, Barauskas O, Xu Y, Fenaux M, et al. (2014). "Inhibition of hepatitis C virus replication by GS-6620, a potent C-nucleoside monophosphate prodrug". Antimicrobial Agents and Chemotherapy. 58 (4): 1930–42. doi:10.1128/AAC.02351-13. PMC 4023746. PMID 24419349.
- ^ Murakami E, Wang T, Babusis D, Lepist EI, Sauer D, Park Y, et al. (2014). "Metabolism and pharmacokinetics of the anti-hepatitis C virus nucleotide prodrug GS-6620". Antimicrobial Agents and Chemotherapy. 58 (4): 1943–51. doi:10.1128/AAC.02350-13. PMC 4023801. PMID 24419340.
- ^ Gentile I, Coppola N, Buonomo AR, Zappulo E, Borgia G (September 2014). "Investigational nucleoside and nucleotide polymerase inhibitors and their use in treating hepatitis C virus". Expert Opinion on Investigational Drugs. 23 (9): 1211–23. doi:10.1517/13543784.2014.921680. PMID 24848437. S2CID 12115460.
- ^ De Clercq E (March 2016). "C-Nucleosides To Be Revisited". Journal of Medicinal Chemistry. 59 (6): 2301–11. doi:10.1021/acs.jmedchem.5b01157. PMID 26513594.
- ^ De Clercq E (November 2019). "New Nucleoside Analogues for the Treatment of Hemorrhagic Fever Virus Infections". Chemistry: An Asian Journal. 14 (22): 3962–3968. doi:10.1002/asia.201900841. PMC 7159701. PMID 31389664.
