| |
| Names | |
|---|---|
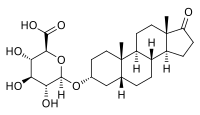
| IUPAC name
17-Oxo-5β-androstan-3α-yl β-D-glucopyranosiduronic acid
| |
| Systematic IUPAC name
(2S,3S,4S,5R,6R)-6-{[(3aS,3bR,5aR,7R,9aS,9bS,11aS)-9a,11a-Dimethyl-1-oxohexadecahydro-1H-cyclopenta[a]phenanthren-7-yl]oxy}-3,4,5-trihydroxyoxane-2-carboxylic acid | |
| Other names
5β-Androstan-3α-ol-17-one 3-glucuronide; 3α-Hydroxy-5β-androstan-17-one 3-glucuronide; Etiocholan-3α-ol-17-one 3-glucuronide; 3α-Hydroxyetiocholan-17-one 3-glucuronide; 17-oxoetiocholan-3α-yl β-D-glucopyranosiduronic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C25H38O8 | |
| Molar mass | 466.571 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Etiocholanolone glucuronide (ETIO-G) is an endogenous, naturally occurring metabolite of testosterone.[1][2] It is formed in the liver from etiocholanolone by UDP-glucuronyltransferases.[1] ETIO-G has much higher water solubility than etiocholanolone and is eventually excreted in the urine via the kidneys.[1][2] Along with androsterone glucuronide, it is one of the major inactive metabolites of testosterone.[3][4]
See also
References
- ^ a b c "Human Metabolome Database: Showing metabocard for Etiocholanolone glucuronide (HMDB0004484)". Hmdb.ca. Retrieved 2022-04-15.
- ^ a b S. Bernstein; S. Solomon (6 December 2012). Chemical and Biological Aspects of Steroid Conjugation. Springer Science & Business Media. pp. 328–. ISBN 978-3-642-95177-0.
- ^ David A. Williams; William O. Foye; Thomas L. Lemke (January 2002). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 707–. ISBN 978-0-683-30737-5.
- ^ Christina Wang (28 May 2007). Male Reproductive Function. Springer Science & Business Media. pp. 69–. ISBN 978-0-585-38145-9.
External links
