| |
| Names | |
|---|---|
| IUPAC name
Diborane(4)
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| 24760 | |
PubChem CID
|
|
| |
| |
| Properties | |
| B2H4 | |
| Molar mass | 25.65 g·mol−1 |
| Related compounds | |
Related compounds
|
Bis(pinacolato)diboron Diboron tetrafluoride Tetrahydroxydiborane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
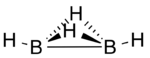
Diborane(4) is a transient inorganic compound with the chemical formula B
2H
4. Stable derivatives are known.
Diborane(4) has been produced by abstraction of two hydrogen atoms from diborane(6) using atomic fluorine and detected by photoionization mass spectrometry.[1] Computational studies predict a structure in which are two hydrogen atoms bridging the two boron atoms via three-centre two-electron bonds in addition to the 2-centre, 2-electron bond between the two boron atoms and one terminal hydrogen atom bonded to each boron atom.[2]
Several stable derivatives of diborane(4) have been reported.[3][4][5]
YouTube Encyclopedic
-
1/5Views:13 2026 1206 5338 2486 813
-
Playing with Self-Igniting Gases: Silane, Phosphine, and Borane | Pyrophoric Chemistry
-
How to Write the Formula for Diboron tetrahydride
-
DIBORANE STRUCTURE / 13 TH GROUP ELEMENTS
-
Deriving diborane molecular orbitals
-
Structure of Diborane & Inorganic Benzene - Boron Family - NEET Chemistry Crash Course
Transcription
References
- ^ Ruščic, B.; Schwarz, M.; Berkowitz, J. (1989). "Molecular structure and thermal stability of B
2H
4 and B
2H+
4 species". The Journal of Chemical Physics. AIP Publishing. 91 (8): 4576–4581. doi:10.1063/1.456745. - ^ Alkorta, Ibon; Soteras, Ignacio; Elguero, José; Del Beneb, Janet E. (23 June 2011). "The boron–boron single bond in diborane(4) as a non-classical electron donor for hydrogen bonding" (PDF). Physical Chemistry Chemical Physics. 13 (31): 14026–14032. Bibcode:2011PCCP...1314026A. doi:10.1039/C1CP20560A. PMID 21698334.
- ^ Xie, Xiaochen; Haddow, Mairi F.; Mansell, Stephen M.; Norman, Nicholas C.; Russell, Christopher A. (2012). "Diborane(4) compounds with bidentate diamino groups". Dalton Transactions. 41 (7): 2140–7. doi:10.1039/C2DT11936F. PMID 22187045.
- ^ Wagner, Arne; Kaifer, Elisabeth; Himmel, Hans-Jörg (2012). "Diborane(4)–metal bonding: Between hydrogen bridges and frustrated oxidative addition". Chemical Communications. 48 (43): 5277–9. doi:10.1039/C2CC31671D. PMID 22526934.
- ^ Horn, Julian; Widera, Anna; Litters, Sebastian; Kaifer, Elisabeth; Himmel, Hans-Jörg (2018). "The proton affinity, HOMO energy and ionization energy of electron-rich sp3–sp3-hybridized diborane(4) compounds with bridging guanidinate substituents can be varied by substitution". Dalton Trans. 47 (6): 2009–2017. doi:10.1039/C7DT04433J. PMID 29345706.
