 | |
| Monoclonal antibody | |
|---|---|
| Type | Whole antibody |
| Source | Chimeric/humanized hybrid (mouse/human) |
| Target | EGFR |
| Clinical data | |
| Other names | ABT-414 |
| ATC code |
|
| Identifiers | |
| CAS Number | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C6624H10228N1728O2052S42 |
| Molar mass | 148251.25 g·mol−1 |
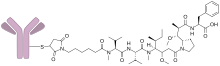
Depatuxizumab mafodotin (INN; development code ABT-414) is an antibody-drug conjugate designed for the treatment of cancer.[1][2] It is composed of an EGFR IGg1 monoclonal antibody (depatuxizumab) conjugated to the tubulin inhibitor monomethyl auristatin F via a stable maleimidocaproyl link.[3][4]
This drug was developed by AbbVie. In May 2019 AbbVie stopped enrolment in all studies of Depatux-M after late-stage failure in newly diagnosed glioblastoma.[5]
In 2014, Orphan Drug Status was granted by the FDA for glioblastoma multiforme.[6] It is in phase II/III clinical trials for glioblastoma, in phase II clinical trials for non-small cell lung cancer, and in phase I clinical trials for the treatment of other solid tumors.[7] Phase I results were presented at ASCO in 2016.[7]
YouTube Encyclopedic
-
1/2Views:449512
-
Miami Global Brain Tumor Symposium
-
09 23 2020 Miami Global Brain Tumor Symposium
Transcription
References
- ^ Statement On A Nonproprietary Name Adopted By The USAN Council - Depatuxizumab Mafodotin, American Medical Association.
- ^ World Health Organization (2016). "International Nonproprietary Names for Pharmaceutical Substances (INN). Proposed INN: List 115" (PDF). WHO Drug Information. 30 (2).
- ^ "Antibody-drug conjugates in the spotlight - The Antibody Society". antibodysociety.org. 14 October 2016.
- ^ Nagayama A, Ellisen LW, Chabner B, Bardia A (December 2017). "Antibody-Drug Conjugates for the Treatment of Solid Tumors: Clinical Experience and Latest Developments". Targeted Oncology. 12 (6): 719–739. doi:10.1007/s11523-017-0535-0. PMID 29116596. S2CID 1012740.
- ^ "AbbVie Provides Update on Depatuxizumab Mafodotin (Depatux-M), an Investigational Medicine for Newly Diagnosed Glioblastoma, an Aggressive Form of Brain Cancer - PM AbbVie, May 17, 2019". abbvie.com. Retrieved 20 May 2019.
- ^ "Depatuxizumab mafodotin". en.pharmacodia.com.
- ^ a b "Depatuxizumab mafodotin - AbbVie - AdisInsight". adisinsight.springer.com.
