| |
| Names | |
|---|---|
| Preferred IUPAC name
Cyclohexanehexathione | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
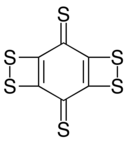
| C6S6 | |
| Molar mass | 264.43 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cyclohexanehexathione is a cyclic covalent compound consisting of a six-carbon ring with a sulfur bonded to each. It has been generated by neutralization of its monoanion (C6S−6) in a mass spectrometer.[1] This compound is the thioketone analog of cyclohexanehexone; that oxygen variant is expected to be substantially less stable.[2] Synthesis of C6S6 by photolysis or pyrolysis to extrude three equivalents of carbon monoxide from a precursor containing adjacent pairs of sulfurs as cyclic dithiocarbonate units gave what is more likely a different valence isomer, as various dithiete-containing structures are predicted to be more stable than the hexathione form.[3]
This theoretical analysis of the various isomers and experimental analysis of this reaction cast doubt on whether the mass spectrometric approach really did produce the hexathione isomer as originally claimed. The increased stability of dithietes as compared to dioxetane-like rings is one of theoretical bases for proposing C6S6 is more stable than the oxygen analog.[2]
References
- ^ Sülzle, Detlev; Beye, Norbert; Fanghänel, Egon; Schwarz, Helmut (1989). "Generation of C6S6 and its radical anion and cation in the gas phase". Chem. Ber. 122 (12): 2411–2412. doi:10.1002/cber.19891221233.
- ^ a b Schröder, Detlef; Schwarz, Helmut; Dua, Suresh; Blanksby, Stephen J.; Bowie, John H. (1999). "Mass spectrometric studies of the oxocarbons CnOn (n = 3–6)". International Journal of Mass Spectrometry. 188 (1–2): 17–25. Bibcode:1999IJMSp.188...17S. doi:10.1016/S1387-3806(98)14208-2. ISSN 1387-3806.
- ^ Nakayama, Juzo; Ishii, Akihiko (2000). Chapter 5: Chemistry of Dithiiranes, 1,2-Dithietanes, and 1,2-Dithietes. Advances in Heterocyclic Chemistry. Vol. 77. Academic Press. pp. 221–284. ISBN 9780080549637.