 | |
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| ECHA InfoCard | 100.034.556 |
| Chemical and physical data | |
| Formula | C20H31NO2S |
| Molar mass | 349.53 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Cetiedil is a vasodilator and an anti-sickling agent.[1]
Synthesis
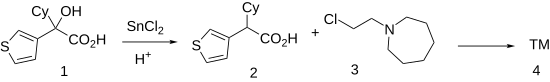
The Clemmensen reduction of 3-thienylcyclohexyl-glycolic acid, CID:11064522 (1) gives cyclohexyl(thiophen-3-yl)acetic acid [16199-74-9] (2). Esterification of the sodium salt of the resulting acid with 1-(2-chloroethyl)azepane [2205-31-4] (3) produces cetiedil (4).
References
- ^ Alavi JB (May 1984). "Sickle cell anemia. Pathophysiology and treatment". The Medical Clinics of North America. 68 (3): 545–56. doi:10.1016/s0025-7125(16)31115-4. PMID 6205230.
- ^ Pons, Robba, FR 1460571 and Pons et al., FR M5504 (1966, 1967, both to Innothera), C.A. 68, 59429d (1968); 71, 91286c (1969).
- ^ Robba, LeGuen, Chim. Ther. 2, 120 (1967).
- ^ Roxburgh, Craig J.; Ganellin, C. Robin; Shiner, Mark A. R.; Benton, David C. H.; Dunn, Philip M.; Ayalew, Yeshi; Jenkinson, Donald H. (1996). "The Synthesis and Some Pharmacological Actions of the Enantiomers of the K+-Channel Blocker Cetiedil". Journal of Pharmacy and Pharmacology. 48 (8): 851–859. doi:10.1111/j.2042-7158.1996.tb03986.x.
- ^ Charles Pigerol, et al. U.S. patent 4,108,865 (1978 to Labaz SA).
- ^ Roxburgh, Craig J.; Ganellin, C. Robin; Athmani, Salah; Bisi, Alessandra; Quaglia, Wilma; Benton, David C. H.; Shiner, Mark A. R.; Malik-Hall, Misbah; Haylett, Dennis G.; Jenkinson, Donald H. (2001). "Synthesis and Structure−Activity Relationships of Cetiedil Analogues as Blockers of the Ca2+-Activated K+Permeability of Erythrocytes†". Journal of Medicinal Chemistry. 44 (20): 3244–3253. doi:10.1021/jm001113w.
