| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
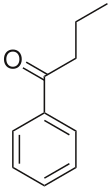
1-Phenylbutan-1-one | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.007.091 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H12O | |||
| Molar mass | 148.20 g/mol | ||
| Appearance | clear liquid | ||
| Melting point | 12 °C (54 °F; 285 K) | ||
| Boiling point | 229 °C (444 °F; 502 K) | ||
| poor | |||
| log P | 2.77 | ||
Refractive index (nD)
|
1.520 | ||
| Hazards | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 99 °C (210 °F; 372 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Butyrophenone is an organic compound with the formula C6H5C(O)C3H7. It is a colorless liquid.
The butyrophenone structure—a ketone flanked by a phenyl ring and a butyl chain—forms the basis for many other chemicals containing various substituents. Some of these butyrophenones are used to treat various psychiatric disorders such as schizophrenia, as well as acting as antiemetics.[1]
Examples of butyrophenone-derived pharmaceuticals include:
- Haloperidol, the most widely used classical antipsychotic drug in this class[1]
- Benperidol, the most potent commonly used antipsychotic (200 times more potent than chlorpromazine)[1][2]
- Lumateperone, an atypical antipsychotic used for schizophrenia and bipolar depression
- Droperidol, Antiemetic for postoperative nausea and vomiting
YouTube Encyclopedic
-
1/5Views:4 8591 4543 7252 088800 607
-
SAR of Fluorobutyrophenone Drugs || L- 10 Unit-4 Medicinal Chemistry -I
-
Pharmacology 301 c AntiPsychotic Drugs Haloperidol Butyrophenone psychoses treatment
-
Brief Notes on Fluorobutyrophenone Category Drugs || L- 11 Unit-4 Medicinal Chemistry -I
-
haloperidol, haldol, haloperidol mechanism of action, haloperidol tablets ip 0.25mg, butyrophenones
-
Pharmacology - ANTIPSYCHOTICS (MADE EASY)
Transcription
References
- ^ a b c Keith Parker; Laurence Brunton Goodman; Louis Sanford; Lazo, John S.; Gilman, Alfred (2006). Goodman & Gilman's The Pharmacological Basis of Therapeutics (11th ed.). New York: McGraw-Hill. ISBN 0071422803.
- ^ Grogan, Charles H.; Rice, Leonard M. (1967). "Ω-Azabicyclic Butyrophenones". Journal of Medicinal Chemistry. 10 (4): 621–623. doi:10.1021/jm00316a022. PMID 6037051.