| Argininosuccinate lyase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 Crystal structure of duck argininosuccinate lyase with bound argininosuccinate.[1] | |||||||||
| Identifiers | |||||||||
| EC no. | 4.3.2.1 | ||||||||
| CAS no. | 9027-34-3 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
The enzyme argininosuccinate lyase (EC 4.3.2.1, ASL, argininosuccinase; systematic name 2-(N ω-L-arginino)succinate arginine-lyase (fumarate-forming)) catalyzes the reversible breakdown of argininosuccinate:
- 2-(N ω-L-arginino)succinate = fumarate + L-arginine
Located in liver cytosol, it is the fourth enzyme of the urea cycle and involved in the biosynthesis of arginine in all species and the production of urea in ureotelic species.[2] Mutations resulting in low activity of the enzyme increase levels of urea in the body and result in various side effects.
The ASL gene is located on chromosome 7 between the centromere (junction of the long and short arm) and the long (q) arm at position 11.2, from base pair 64,984,963 to base pair 65,002,090.
ASL is related to intragenic complementation.[3][4][5]
YouTube Encyclopedic
-
1/3Views:276 5183 5692 478
-
Six types of enzymes | Chemical Processes | MCAT | Khan Academy
-
Añadir archivos .ASL
-
Mod-01 Lec-22 Urea Cycle, Gluconeogenesis and Glyoxalate Cycle
Transcription
So today, we're going to talk about enzymes and all the different kinds of reactions that enzymes can catalyze. But before we do that, let's review the idea that enzymes make biochemical reactions go faster. And if you look at a reaction coordinate diagram, you'd notice that enzymes speed up reactions by lowering their activation energy. Now, enzymes are generally named for their reactions, which is convenient because it makes it a lot easier to remember what an enzyme does if someone gives you its name. And a great example of this is that one of the enzymes involved in DNA replication is called DNA polymerase, which is named as such because it acts on DNA and specifically makes polymers of DNA. Now, the suffix "ase" is usually just one that you find at the end of most enzyme names. Now, another great example is that the enzyme that catalyzes the first step of glycolysis, which you may remember is the reaction between glucose and ATP to form glucose-6-phosphate and ADP, is called hexokinase. And "hexo" refers to the number 6, which is a reference to glucose being a six-carbon sugar. And "kinase" is a term referring to enzymes that add phosphate functional groups to different substrates. So overall, hexokinase adds phosphates to six-carbon sugars like glucose. Now generally, every enzyme has a very specific name that gives insight into the specific reaction that that enzyme can catalyze. So we can actually divide most enzymes into six different categories based off the kinds of reactions that they catalyze. Now, our first group is the transferase group. And the basic reaction that transferases catalyze are ones where you move some functional group, X, from molecule B to molecule A. And a great example of one of these reactions occurs during protein translation, where amino acids bound to tRNA molecules are transferred over to the growing polypeptide chain. So in this case, A refers to our amino acid chain, B refers to our tRNA, and X refers to this lysine residue, which is being transferred from B to A. And this reaction in particular is catalyzed by an enzyme called peptidyl transferase, which is an appropriate name since it is a transferase involved in making peptides. Next we have the ligase group, which catalyzes reactions between two molecules, A and B, that are combining to form a complex between the two, or AB. And an example of a reaction using a ligase that you might be familiar with occurs during DNA replication, where two strands of DNA are being joined together. So in this reaction, A and B represent the two separated DNA polymers, which are being joined to form a single strand. And this reaction in particular is catalyzed by an enzyme called DNA ligase, which is named since it's a ligase that works on DNA strands. Now our third group as the oxidoreductase group, which is a little different from the others since it actually includes two different types of reactions. And these reactions involve transferring electrons from either molecule B to molecule A or from molecule A to molecule B. Now, we say that an oxidase is directly involved in oxidizing or taking electrons away from a molecule, while a reductase is involved in reducing or giving electrons to a molecule. And we call these enzymes oxidoreductases together because they can usually catalyze both the forward and reverse reactions, which is why I used equilibrium arrows here instead of just a normal single-headed arrow. Now a great example of an oxidation reduction reaction occurs during lactic acid fermentation, where electrons are either passed from NADH to pyruvate or from lactic acid to NAD. Now, this reaction is catalyzed by an enzyme called lactate dehydrogenase. Remember that the word "dehydrogenase" refers to the removal of a hydride functional group. And that's the same as saying the removal of electrons, since hydrides are basically just hydrogen atoms with two electrons on them instead of just one. Now, this enzyme is given its name since it's able to remove a hydride, or remove electrons, from a molecule of lactic acid. Next, we have the isomerase group. And enzymes in this group are typically involved in reactions where a molecule, like molecule A, is being converted to one of its isomers. And an example of this type of a reaction is the conversion of glucose-6-phostate to fructose-6-phosphate, which is one of the steps of glycolysis that you may remember. Now, this reaction is catalyzed by an enzyme called phosphoglucose isomerase, which is appropriately named since it creates isomers of glucose molecules that are phosphorylated. Now, our next category is the hydrolase category. And hydrolases use water to cleave a molecule, like molecule A, into two other molecules, B and C. And a great example of one of these reactions is the hydrolysis reaction that can occur to peptide bonds. And if we have this lysine-alanine dipeptide here, it could be reacted with water to form two individual amino acids that are no longer bound. And this particular hydrolysis reaction can be catalyzed by a class of enzymes that we call serine hydrolases, which some people call serine proteases. And they are named this way because they are hydrolases that use a serine residue as the key catalytic amino acid that is responsible for breaking the peptide bond. Now, our last category is a little more complicated than the others. And it's the lyase group. Now, lyases catalyze the dissociation of a molecule, like molecule A, into molecule B and C, without using water like hydrolases would, and without using oxidation or reduction like an oxidoreductase would. And one example of a reaction catalyzed by a lyase is the cleavage of argininosuccinate into arginine and succinate. And this reaction takes place during the urea cycle, which you also might be familiar with. Now this specific reaction is catalyzed by an enzyme called argininosuccinate lyase, which is appropriately named because it is a lyase that catalyzes the breakdown of an argininosuccinate molecule. Now, it's important to recognize that since lyases don't use water or oxidation to break a bond, they need to generate either a double bond between two atoms or a ring structure in a molecule in order to work. So what did we learn? Well, first we learned that enzymes are sometimes named for their reactions. And next we learned about the six different types of enzymes. We have transferases, which transfer functional groups from one molecule to another; ligases, which ligate or join two molecules together; oxidoreductases, which move electrons between molecules; isomerases, which convert a molecule from one isomer to another; hydrolases, which break bonds using water; and lyases, which break bonds without using water and without using oxidation.
Structure
ASL is composed of four identical monomers; each monomer consisting of a single polypeptide chain between 49 and 52 kDa,[6] between 196 and 208 kDa for the entire tetrameric enzyme. Each monomer has three highly conserved regions remote from one another, but these regions cluster together in the tetramer to form four active sites. Therefore, each ASL homotetramer has four active sites to catalyze the breakdown of argininosuccinate.
Each monomer in the ASL homotetramer is composed of three structural domains; all three are primarily alpha helical. Domains 1 and 3 are similar in structure as they both consist of helix-turn-helix motifs. Domain 1 of the monomer contains the amino terminus. Domain 2 contains one small beta sheet, nine alpha helices, and the carboxyl terminus. Three of the nine alpha helices on one monomer are engaged mainly in hydrophobic interactions with another monomer to form a dimer. Two dimers then associate by way of alpha helix, one from each monomer, to form a central 20-helix core. The association of all four monomers allows for the catalytic activity at each possible active site.[4]
Intragenic complementation
Multiple copies of a polypeptide encoded by a gene often can form an aggregate referred to as a multimer. When a multimer is formed from polypeptides produced by two different mutant alleles of a particular gene, the mixed multimer may exhibit greater functional activity than the unmixed multimers formed by each of the mutants alone. When a mixed multimer displays increased functionality relative to the unmixed multimers, the phenomenon is referred to as intragenic complementation. In humans, ASL is a multimer (tetramer) protein. An ASL disorder in humans can arise from mutations in the ASL gene, particularly mutations that affect the active site of the mutant multimer protein. ASL disorder is associated with considerable clinical and genetic heterogeneity which is considered to reflect the extensive intragenic complementation occurring among individual patients.[3][4][5]
Mechanism
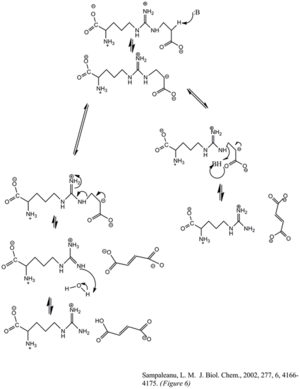

The enzyme's cleavage of the argininosuccinate, to form fumarate and arginine, occurs through an E1cb elimination reaction. The base initiates the reaction by deprotonating the carbon adjacent to the arginine, or leaving group. Recent mutagenic studies of ASL homologues have shown that Histidine 162 or Threonine 161 of ASL is responsible for the proton abstraction of the Cβ, either directly or indirectly through a water molecule.[6] Lysine 289 is thought to stabilize the negatively charged carbanion intermediate. Although there is no consensus of the catalytic acid that donates the proton to the imine functional group of the arginine product, some mutagenesis studies show serine 283 may be involved.[6]
Role in the urea cycle
Ammonia (NH3) is a toxic substance for many aerobic organisms and must be excreted. Some aquatic organisms release the toxin right directly into their environment, while other ureotelic species must convert their toxic nitrogen waste into non-toxic components, like uric acid or urea, through a series of catalyzed steps better known as the urea cycle. ASL catalyzes the fourth step in the cycle, following the action of argininosuccinate synthetase (ASS) in the liver cytosol. While ASS catalyzes the formation of argininosuccinate from citrulline and aspartate, ASL breaks the newly formed argininosuccinate into L-arginine and fumarate. L-arginine continues through the urea cycle to form urea and ornithine, while fumarate can enter the citric acid cycle.[7]
δ-Crystallin
ASL, δ-crystallin, class II fumarase, aspartase, adenylosuccinase lyase, and 3-carboxy-cis and cis-muconate lactonizing enzyme are all members of the same homotetrameric superfamily of enzymes, in which most catalyze the same type of elimination reactions where a C-O or C-N bond is broken and fumarate is released as a product. δ-crystallins are the major structural eye lens water-soluble proteins of most birds, reptiles, and some other vertebrates.[4]
Within the superfamily, ASL is most closely related to δ-crystallin in amino acid sequence and in protein fold structure. There are two isoforms of δ-crystallin, δI and δII. These two isoforms conserve 69% and 71% of the ASL amino acid sequence, respectively, but only the δII isoform retains the same enzymatic activity as ASL. The similarities have led researches to believe that these crystallins have evolved from the recruitment to the lens of preexisting metabolic enzymes, like ASL, by a process called 'gene sharing'. The same gene product functions as both a lens crystallin and an enzyme in other non-ocular tissues. Comparative studies of the δ-crystallins have been beneficial for understanding the enzymatic mechanism of the ASL reaction.[8]
Mutations and ASL deficiencies: argininosuccinic aciduria
Mutations in the human ASL gene causes argininosuccinic aciduria, a rare autosomal recessive disorder, and results in deficiencies of the urea cycle. Argininosuccinate lyase is an intermediate enzyme in the urea synthesis pathway and its function is imperative to the continuation of the cycle. A non-functioning enzyme results in patients' accumulation of ammonia, argininosuccinate, and citrulline in the blood, and argininosuccinate is excreted in the urine.[9] Other resulting symptoms include lethargy, vomiting, hypothermia, hyperventilation, hepatomegaly and progressive encephalopathy in infant patients, and abnormal hair growth, hepatic fibrosis, episodic vomiting, growth and developmental delay,[9] in patients experiencing the disorder later in childhood.
ASL is a key enzyme in the conversion of ammonia to urea through the urea cycle. Ammonia builds to toxic levels, resulting in hyperammonemia.[10] Ammonia is toxic in part because it affects the nervous system. There is biochemical evidence that shows rises in ammonia can inhibit glutaminase and therefore limit the rate of synthesis of neurotransmitters such as glutamate,[11] which can explain the developmental delay in argininosuccinic aciduria patients.
One mutation in patients with argininosuccinic aciduria occurs when glutamine 286 is mutated to arginine. The enzyme now has a positively charged arginine in place of a neutrally charged glutamine and studies suggest this change may sterically and/or electrostatically hinder a conformational change necessary for catalysis.
References
- ^ PDB: 1TJW; Sampaleanu LM, Codding PW, Lobsanov YD, Tsai M, Smith GD, Horvatin C, Howell PL (December 2004). "Structural studies of duck delta2 crystallin mutants provide insight into the role of Thr161 and the 280s loop in catalysis". Biochem. J. 384 (Pt 2): 437–47. doi:10.1042/BJ20040656. PMC 1134128. PMID 15320872.
- ^ a b PDB: 1K62; Sampaleanu LM, Vallée F, Thompson GD, Howell PL (December 2001). "Three-dimensional structure of the argininosuccinate lyase frequently complementing allele Q286R". Biochemistry. 40 (51): 15570–80. doi:10.1021/bi011525m. PMID 11747432.
- ^ a b Turner MA, Simpson A, McInnes RR, Howell PL (August 1997). "Human argininosuccinate lyase: a structural basis for intragenic complementation". Proc. Natl. Acad. Sci. U.S.A. 94 (17): 9063–8. Bibcode:1997PNAS...94.9063T. doi:10.1073/pnas.94.17.9063. PMC 23030. PMID 9256435.
- ^ a b c d Yu B, Howell PL (October 2000). "Intragenic complementation and the structure and function of argininosuccinate lyase". Cell. Mol. Life Sci. 57 (11): 1637–51. doi:10.1007/PL00000646. PMID 11092456. S2CID 1254964.
- ^ a b Yu B, Thompson GD, Yip P, Howell PL, Davidson AR (December 2001). "Mechanisms for intragenic complementation at the human argininosuccinate lyase locus". Biochemistry. 40 (51): 15581–90. doi:10.1021/bi011526e. PMID 11747433.
- ^ a b c Sampaleanu LM, Yu B, Howell PL (February 2002). "Mutational analysis of duck delta 2 crystallin and the structure of an inactive mutant with bound substrate provide insight into the enzymatic mechanism of argininosuccinate lyase". J. Biol. Chem. 277 (6): 4166–75. doi:10.1074/jbc.M107465200. PMID 11698398.
- ^ Pratt, Charlotte Amerley; Voet, Donald; Voet, Judith G. (2008). "Figure 20.8". Fundamentals of biochemistry: life at the molecular level. New York: Wiley. ISBN 978-0-470-12930-2.
- ^ Chakraborty AR, Davidson A, Howell PL (February 1999). "Mutational analysis of amino acid residues involved in argininosuccinate lyase activity in duck delta II crystallin". Biochemistry. 38 (8): 2435–43. doi:10.1021/bi982150g. PMID 10029537.
- ^ a b Ficicioglu C, Mandell R, Shih VE (November 2009). "Argininosuccinate lyase deficiency: longterm outcome of 13 patients detected by newborn screening". Mol. Genet. Metab. 98 (3): 273–7. doi:10.1016/j.ymgme.2009.06.011. PMC 2773214. PMID 19635676.
- ^ "ASL gene argininosuccinate lyase". NIH. U.S. Department of Health & Human Services. 2007.
- ^ Jack, JJB (1982). "Actions of ammonia on the central nervous system". Journal of Inherited Metabolic Disease. 5 (S2): 104. doi:10.1007/BF01805572. S2CID 33915515.
External links
- GeneReviews/NCBI/NIH/UW entry on Urea Cycle Disorders Overview
- GeneReviews/NCBI/NIH/UW entry on Argininosuccinate Lyase Deficiency
- OMIM entries on Argininosuccinate Lyase Deficiency
- GeneCard

