Alfred Wohl | |
|---|---|
 | |
| Born | 3 October 1863 |
| Died | 25 December 1939 (aged 76) |
| Alma mater | University of Heidelberg |
| Known for | Wohl degradation, Wohl–Aue reaction, Wohl–Ziegler reaction |
| Scientific career | |
| Institutions | University of Berlin, Technical University of Danzig |
| Doctoral advisor | August Wilhelm von Hofmann |
Alfred Wohl (3 October 1863 – 25 December 1939) was a German chemist. Several chemical reactions are named after him, including the Wohl degradation, Wohl–Aue reaction and the Wohl–Ziegler reaction.
YouTube Encyclopedic
-
1/3Views:596439 01174 633
-
Wohl Ziegler Bromination - Named Reactions in Organic Chemistry
-
Protein: Chemistry for Understanding Nutrition by Milton Mills, MD
-
Early forensics and crime-solving chemists - Deborah Blum
Transcription
Life
Wohl studied chemistry at the University of Heidelberg from 1882 until 1886. He received his Ph.D 1886 for work on Hexamethylenetetramine with August Wilhelm von Hofmann. He became an assistant of Hermann Emil Fischer at the University of Berlin from 1886 until 1891, where he also received his habilitation. He became professor at the University of Berlin in 1901, but he left for the Technical University of Danzig in 1904. He retired because of antisemitic pressure in 1933, but worked in his lab until 1937. He emigrated to Sweden in 1938, where he died in 1939.
His son Kurt Wohl (1896–1962) – who also became a renowned chemical scientist – emigrated to Great Britain in 1939 and some years later to the United States.[1]
Work
His work in organic chemistry started with his PhD thesis on hexamine in 1886. Under the influence of Fischer, Wohl focused on sugar chemistry. Wohl arbitrarily defined the structure of (+)-glyceraldehyde to have the D-configuration, forming the basis for the D-L system. This was done before chemists had the ability to prove (+)-glyceraldehyde was, in fact, D-glyceraldehyde. Before the invention of x-ray crystallography the exact determination of the configuration at a chiral carbon atom was impossible.
| D-glyceraldehyde |
L-glyceraldehyde |
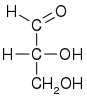
|
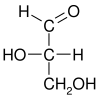
|
With this starting point, all related chiral compounds could be chemically transformed into (−)- or (+)-glyceraldehyde. By employing only chemical transformations with retention of stereochemical configuration, such an unknown chiral compound could be assigned either a D- or an L-configuration.
The use of vanadium pentoxide for the catalytic oxidation with air of various substances became his most profitable invention. Similar catalysts are still used for the oxidation of naphthalene, anthraquinone and for the production of sulfuric acid from sulfur dioxide.
References
- ^ Kurt Wohl – His Life and Work www.researchgate.net, January 2003
- Teresa Sokolowska, Romuald Piosik (2004). "Otto Ruff und Alfred Wohl. Professoren der 1904 gegründeten Königlichen Technischen Hochschule zu Danzig". Chemkon. 11 (2): 76–78. doi:10.1002/ckon.200410006.
