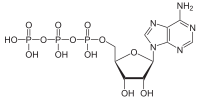
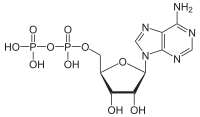

ATP hydrolysis is the catabolic reaction process by which chemical energy that has been stored in the high-energy phosphoanhydride bonds in adenosine triphosphate (ATP) is released after splitting these bonds, for example in muscles, by producing work in the form of mechanical energy. The product is adenosine diphosphate (ADP) and an inorganic phosphate (Pi). ADP can be further hydrolyzed to give energy, adenosine monophosphate (AMP), and another inorganic phosphate (Pi).[1] ATP hydrolysis is the final link between the energy derived from food or sunlight and useful work such as muscle contraction, the establishment of electrochemical gradients across membranes, and biosynthetic processes necessary to maintain life.
Anhydridic bonds are often labelled as "high-energy bonds". P-O bonds are in fact fairly strong (~30 kJ/mol stronger than C-N bonds)[2][3] and themselves not particularly easy to break. As noted below, energy is released by the hydrolysis of ATP. However, when the P-O bonds are broken, input of energy is required. It is the formation of new bonds and lower-energy inorganic phosphate with a release of a larger amount of energy that lowers the total energy of the system and makes it more stable.[1]
Hydrolysis of the phosphate groups in ATP is especially exergonic, because the resulting inorganic phosphate molecular ion is greatly stabilized by multiple resonance structures, making the products (ADP and Pi) lower in energy than the reactant (ATP). The high negative charge density associated with the three adjacent phosphate units of ATP also destabilizes the molecule, making it higher in energy. Hydrolysis relieves some of these electrostatic repulsions, liberating useful energy in the process by causing conformational changes in enzyme structure.
In humans, approximately 60 percent of the energy released from the hydrolysis of ATP produces metabolic heat rather than fuel the actual reactions taking place.[4] Due to the acid-base properties of ATP, ADP, and inorganic phosphate, the hydrolysis of ATP has the effect of lowering the pH of the reaction medium. Under certain conditions, high levels of ATP hydrolysis can contribute to lactic acidosis.
YouTube Encyclopedic
-
1/3Views:117 121333 28844 382
-
ATP hydrolysis: Gibbs free energy | Biomolecules | MCAT | Khan Academy
-
ATP hydrolysis mechanism | Energy and enzymes | Biology | Khan Academy
-
Mechanism of ATP Hydrolysis
Transcription
So let's talk about one of the most famous molecules in all of biochemistry, ATP. So why is ATP so famous? Well, it's the energy currency of the cell. And the reason it's called the energy currency of the cell is because it powers, it essentially fuels, many life sustaining reactions inside of our body. So some examples of these include biosynthesis of biomolecules. So remember fats, proteins, carbohydrates, and nucleic acids are all essential to life, and building these molecules requires energy in the form of ATP. In addition, ATP is also used to contract our muscles. And this is very important in order to allow living organisms to move. And additionally, ATP is also involved in some ion movement across cell membranes. And of course, moving ions across membranes is really important to maintain a comfortable internal environment within the cell. Now, you could take my word for it that somehow ATP magically powers all of these life-sustaining processes, but you really don't have to. In fact, in this video, we're going to review some topics from general chemistry to really understand how ATP, on a chemical level, really fuels these reactions. Now, the topic we want to review in introductory chemistry is a thermodynamic parameter called Gibbs free energy, or as it's more often written as just simply delta G. So now recall that delta G is a quantitative number. And it's a number that's measured in units of joules, which is a measurement of energy. And depending on whether this value is positive or negative, it tells us whether or not a reaction requires energy, or whether a reaction releases energy. Now remember that delta G is equal to the free energy of the products of a reaction, minus the free energy of the reactants in a reaction. So if the change in Gibbs free energy is negative, which means that the products have a much smaller free energy than the reactant, we say that this reaction releases energy. On the other hand, if we have a positive value of delta G, which means that our products are at a much higher energy level than our reactants, we say that that reaction requires an input of energy. And I know I've been kind of nebulous about this term energy here. And so, briefly, I want to remind you that the change in free energy going from reactants to products of a reaction takes into account both the change in enthalpy, as well as the change in entropy, which are two topics that you might be familiar with from general chemistry. So how does this all relate to ATP? Well, it turns out that there is a reaction involving ATP that has a very large negative delta G value. That is to say it releases a lot of free energy. Specifically, this reaction involves ATP combining with water, and when it combines with water, we call this a hydrolysis reaction. So I'll just write that here to remind us. And the products of this reaction are a molecule called ADP and a free phosphate group. And like I mentioned before, the change in Gibbs free energy is very negative. So what's going on here? So ATP starts out with triphosphate, three phosphate groups, loses a phosphate group because it becomes diphosphate. And then it forms a free phosphate group that it cleaved off. So on first glance, it might seem that this reaction is not balanced because we don't have this hydrogen or oxygen on the right side of our equation. But I just want to note here that the negatively charged hydroxyl group becomes a part of the phosphate group. And the remaining hydrogen ion of the water combines with another molecule of water in solution to become a positively charged hydronium ion. And usually, these two things are left out just for the sake of convenience. But I wanted to point them out here so that you wouldn't be confused by the stoichiometry. On the other hand, many biosynthesis reactions in the body have a positive delta G value. So remember, a positive delta G value means it requires an input of energy. And an example of this type of reaction is when we take a monomer, such as an amino acid for example, and we string them together covalently to form a polymer. So in the case of an amino acid, that would mean we're forming a long peptide chain. Now here's where our knowledge of introductory chemistry comes in. So in thermodynamics, the study of energy changes, there's an important principle that states that the overall delta G for a reaction-- I'm going to scroll down here to give us some more space. So the overall delta G for a reaction is equal to the sum of the delta G values for the individual steps of a reaction. So let's actually go ahead and add these two reactions together and see what happens. So let's write that out. So we have ATP as a reactant, as well as water, as well as our monomer subunits. And we are producing ADP, a free phosphate group, and a polymer. Now what is the delta G for our overall reaction? Well, we just simply have to add the delta G values for each step. Now I didn't give you actual numerical values for each of these steps. But in general, the hydrolysis of ATP produces energy in excess of the energy needed for biosynthesis reactions, such as this one. So essentially what I'm saying is that if we add a very large negative number to a smaller positive number, we will get an overall negative delta G value. In other words, we have just taken a previously energetically unfavorable reaction with a positive delta G value and turned it into an energetically favorable reaction with a negative delta G value. And we have done this by what we call coupling a reaction that has a favorable delta G value, such as the ATP hydrolysis, with a reaction that has an unfavorable delta G value. I want to mention that the ability to add these delta G values tells us nothing about the path that the reaction actually takes. And in fact, generally speaking, almost never does a reaction proceed in two discrete steps like it's written here. Instead, this coupled process often occurs simultaneously. But as you can see, it's still beneficial to separate these two reactions into two discrete steps, so you can prove to yourself essentially why ATP, with its negative delta G value, is able to fuel energetically unfavorable processes.
Amount of energy produced
Hydrolysis of the terminal phosphoanhydridic bond is a highly exergonic process. The amount of released energy depends on the conditions in a particular cell. Specifically, the energy released is dependent on concentrations of ATP, ADP and Pi. As the concentrations of these molecules deviate from values at equilibrium, the value of Gibbs free energy change (ΔG) will be increasingly different. In standard conditions (ATP, ADP and Pi concentrations are equal to 1M, water concentration is equal to 55 M) the value of ΔG is between -28 and -34 kJ/mol.[5][6]
The range of the ΔG value exists because this reaction is dependent on the concentration of Mg2+ cations, which stabilize the ATP molecule. The cellular environment also contributes to differences in the ΔG value since ATP hydrolysis is dependent not only on the studied cell, but also on the surrounding tissue and even the compartment within the cell. Variability in the ΔG values is therefore to be expected.[6]
The relationship between the standard Gibbs free energy change ΔrGo and chemical equilibrium is revealing. This relationship is defined by the equation ΔrGo = -RT ln(K), where K is the equilibrium constant, which is equal to the reaction quotient Q in equilibrium. The standard value of ΔG for this reaction is, as mentioned, between -28 and -34 kJ/mol; however, experimentally determined concentrations of the involved molecules reveal that the reaction is not at equilibrium.[6] Given this fact, a comparison between the equilibrium constant, K, and the reaction quotient, Q, provides insight. K takes into consideration reactions taking place in standard conditions, but in the cellular environment the concentrations of the involved molecules (namely, ATP, ADP, and Pi) are far from the standard 1 M. In fact, the concentrations are more appropriately measured in mM, which is smaller than M by three orders of magnitude.[6] Using these nonstandard concentrations, the calculated value of Q is much less than one. By relating Q to ΔG using the equation ΔG = ΔrGo + RT ln(Q), where ΔrGo is the standard change in Gibbs free energy for the hydrolysis of ATP, it is found that the magnitude of ΔG is much greater than the standard value. The nonstandard conditions of the cell actually result in a more favorable reaction.[7]
In one particular study, to determine ΔG in vivo in humans, the concentration of ATP, ADP, and Pi was measured using nuclear magnetic resonance.[6] In human muscle cells at rest, the concentration of ATP was found to be around 4 mM and the concentration of ADP was around 9 μM. Inputing these values into the above equations yields ΔG = -64 kJ/mol. After ischemia, when the muscle is recovering from exercise, the concentration of ATP is as low as 1 mM and the concentration of ADP is around 7 μM. Therefore, the absolute ΔG would be as high as -69 kJ/mol.[8]
By comparing the standard value of ΔG and the experimental value of ΔG, one can see that the energy released from the hydrolysis of ATP, as measured in humans, is almost twice as much as the energy produced under standard conditions.[6][7]
See also
References
- ^ a b Lodish, Harvey (2013). Molecular cell biology (7th ed.). New York: W.H. Freeman and Co. pp. 52, 53. ISBN 9781464109812. OCLC 171110915.
- ^ Darwent, B. deB. (1970). "Bond Dissociation Energies in Simple Molecules", Nat. Stand. Ref. Data Ser., Nat. Bur. Stand. (U.S.) 31, 52 pages.
- ^ "Common Bond Energies (D". www.wiredchemist.com. Retrieved 2020-04-04.
- ^ Berne & Levy physiology. Berne, Robert M., 1918-2001., Koeppen, Bruce M., Stanton, Bruce A. (6th, updated ed.). Philadelphia, PA: Mosby/Elsevier. 2010. ISBN 9780323073622. OCLC 435728438.
{{cite book}}: CS1 maint: others (link) - ^ "Standard Gibbs free energy of ATP hydrolysis - Generic - BNID 101989". bionumbers.hms.harvard.edu. Retrieved 2018-01-25.
- ^ a b c d e f Philips, Ron Milo & Ron. "» How much energy is released in ATP hydrolysis?". book.bionumbers.org. Retrieved 2018-01-25.
- ^ a b "ATP: Adenosine Triphosphate". cnx.org. 21 October 2016. Retrieved 2018-05-16.
- ^ Wackerhage, H.; Hoffmann, U.; Essfeld, D.; Leyk, D.; Mueller, K.; Zange, J. (December 1998). "Recovery of free ADP, Pi, and free energy of ATP hydrolysis in human skeletal muscle". Journal of Applied Physiology. 85 (6): 2140–2145. doi:10.1152/jappl.1998.85.6.2140. ISSN 8750-7587. PMID 9843537. S2CID 2265397.
Further reading
- Syberg, F.; Suveyzdis, Y.; Kotting, C.; Gerwert, K.; Hofmann, E. (2012). "Time-Resolved Fourier Transform Infrared Spectroscopy of the Nucleotide-binding Domain from the ATP-binding Cassette Transporter MsbA: ATP Hydrolysis ID The Rate-Limiting Step in the Catalytic Cycle". Journal of Biological Chemistry. 278 (28): 23923–23931. doi:10.1074/jbc.M112.359208. PMC 3390668. PMID 22593573.
- Zharova, T. V.; Vinogradov, A. D. (2003). "Proton-Translocating ATP-synthase of Paracoccus denitrificans: ATP- Hydrolytic Activity". Biochemistry. Moscow. 68 (10): 1101–1108. doi:10.1023/A:1026306611821. PMID 14616081. S2CID 19570212.
- Kamerlin, S. C.; Warshel, A. (2009). "On the energetics of ATP hydrolysis in solution". Journal of Physical Chemistry. B. 113 (47): 15692–15698. doi:10.1021/jp907223t. PMID 19888735.
- Bergman, C.; Kashiwaya, Y.; Veech, R. L. (2010). "The effect of pH and Free Mg2+ on ATP Linked Enzymes and the Calculation of Gibbs Free Energy of ATP Hydrolysis". Journal of Physical Chemistry. 114 (49): 16137–16146. doi:10.1021/jp105723r. PMID 20866109.
- Berg, J. M.; Tymoczko, J. L.; Stryer, L. (2011). Biochemistry (International ed.). New York: W. H. Freeman. p. 287.
