| |
| Names | |
|---|---|
| Other names
AlCp*, Cp*Al
| |
| Identifiers | |
| |
| Properties | |
| C10H15Al | |
| Molar mass | 162.212 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
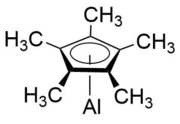
(Pentamethylcyclopentadienyl)aluminium(I) is an organometallic compound with the formula Al(C5Me5) ("Me" is a methyl group; CH3). The compound is often abbreviated to AlCp* or Cp*Al, where Cp* is the pentamethylcyclopentadienide anion (C5Me5−). Discovered in 1991 by Dohmeier et al.,[1] AlCp* serves as the first ever documented example of a room temperature stable monovalent aluminium compound. In its isolated form, Cp*Al exists as the tetramer [Cp*Al]4, and is a yellow crystal that decomposes at temperatures above 100 °C but also sublimes at temperatures above 140 °C.[1][2]
Synthesis
The earliest documented synthesis and characterization of Cp*Al was by Dohmeier et al. in 1991,[1] where four equivalents of AlCl in toluene/diethyl ether is reacted with two equivalents of 2[Mg(Cp*)2] to give [Cp*Al]4 as yellow crystals:
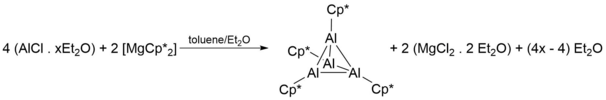
Despite the above synthetic scheme successfully producing tetrameters of [Cp*Al]4 at reasonable yields (44%), its use of AlCl proved problematic, as AlCl synthesis requires harsh conditions and its reactive nature makes storage a challenge. As such, more facile ways of synthesising the [Cp*Al]4 tetramer were discovered, and required the reduction of Cp*AlX2 (X = Cl, Br, I) by a metal (K when X = Cl) or a metal alloy (Na/K alloys when X = Br, I):[3][4][5][6][7]

More exotic ways of synthesizing [Cp*Al]4 include the controlled disproportionation of an Al(II) dialane into constituent Al(I) and Al(III) products. For example, reacting dialane [Cp*AlBr]2 with a Lewis base such as pyridine the Lewis base stabilized [Cp*AlBr2] and [Cp*Al]4.[8]
Monomeric Cp*Al has also been isolated in a solid Ar matrix by heating [Cp*Al]4 in toluene to 133 °C and spraying the resultant vapours with Ar onto a copper block kept at 12 K.[9]
Structure and bonding
X-ray crystallographic data determined Cp*Al to exist exclusively as a tetramer in its solid state. This tetramer, [Cp*Al]4, consists of an Al4 tetrahedron, and the Cp* rings are ŋ5-coordinated to the aluminium(I) cation such that the planes of the C5Me5- rings are approximately parallel to the opposite base of the Al4 tetrahedron.[1] The perpendicular distance between Al and the Cp* ring was determined through crystallography to range from 199.7 to 203.2 pm, with a mean value of 201.5 pm.[1] The Al-Al bond in [Cp*Al]4 is 276.9 pm, which is slightly shorter than that of metallic aluminium, which has an Al-Al bond length of 286 pm.[1] Additionally, the Al-Al bond in [Cp*Al]4 is significantly shorter than other oligomeric and polymeric Group III M(I)-ŋ5-Cp* compounds such as octahedral [InCp*]6 (394, 336 pm), dimeric [InCp*]2 (363.1 pm), and polymeric [TlCp*] (641 pm), indicating a significantly larger interaction between aluminium atoms in [Cp*Al]4 than monovalent Cp* compounds of In(I) and Tl(I).[3] Additional characterization that has been performed include Raman spectroscopy, which detected a Raman active breathing vibration (A1, 377 cm-1) of the Al4 tetrahedron in [Cp*Al]4.[1]
Natural bond orbital (NBO) analysis of [Cp*Al] and [Cp*Al]4 using B3LYP/6-31G(d,p) calculated the average charge transfer per Cp* fragment to an Al atom to be 0.657 and 0.641 respectively. This is slightly higher than the charge transfers calculated on [CpAl] and [Cp*Al]4 (0.630 and 0.591 respectively). NBO calculation of the HOMO-LUMO gap in [Cp*Al] also revealed a significant decreasing in the tetrameric [Cp*Al]4 complex compared to the monomeric [Cp*Al] (4.36 compared to 5.49), which is consistent with density functional theory calculations of analogous systems including superatom complexes of gold, aluminium and gallium.[10] Atoms in molecules (AIM) calculations calculate the Al-Al bonding to be metallic.[11] Stabilization of [Cp*Al]4 relative to [CpAl]4 is thought to arise from addition of H-H interactions on the methyl groups attached to the Cp* ligand as opposed to the increased Al-Al bonding interactions.[11]
Despite its typically tetrameric form, the monomer Cp*Al has been isolated and studied in the gas-phase using gas-phase electron diffraction. In its gaseous monomeric form, the perpendicular distance between the Al to the Cp* ring was calculated to be 206.3(8) pm, which is slightly longer than tetrameric [Cp*Al]4.[2]
Reactivity
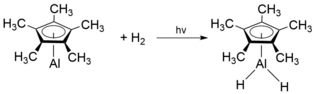
When isolated in a solid H2 doped Ar matrix, monomeric Cp*Al has shown to form the hydride species H2Cp*Al upon exposure to H2 and photolysis with a Hg lamp:[9]
At temperatures above 100 °C, [Cp*Al]4 decomposes to form pentamethylcyclopentandiene (Cp*H), metallic aluminium (Al(0)) and other non-volatile Al(III) compounds.[2] The overall stability of [Cp*Al]4 is unique as there is a thermodynamic affinity for tetrameric aluminium(I) compounds ([RAl]4) to disproportionate into elemental aluminium and R3Al. As such, a number of different novel oligomeric structures can be synthesised when using tetrameric [Cp*Al]4 as a precursor.[6] For example, treatment of [Cp*Al]4 with excess selenium and tellurium in mild conditions gives the unique heterocubane structures [Cp*AlSe]4 and [Cp*AlTe]4 respectively.[4] These heterocubane structures are extremely air and moisture sensitive, leading to its decomposition and evolution of H2Se and H2Te respectively. Analogously, reaction of [Cp*Al]4 with lighter chalcogens such as O2, N2O and sulfur yield [Cp*AlX]4 (X = O, S).[12]
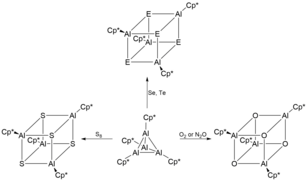
[Cp*Al]4 was also the used as a precursor to synthesize the first ever stable dimeric iminoalane containing an Al2N2 heterocycle through the treatment of [Cp*Al]4 with Me3SiN3 in a 1:4 molar ratio.[13] The resultant iminoalanes was characterized to contain an ideally planar Al2N2 core ring with three coordinate aluminium and nitrogen atoms. Other dimeric iminoalanes including [Cp*AlNSi(i-Pr)3]2, [Cp*AlNSiPh3]2 and [Cp*AlNSi(t-Bu)3]2 have since been synthesized using [Cp*Al]4 as a precursor through oxidative addition of an organic azide.[3]

Function as a ligand

[Cp*Al] is able to act as an atypical exotic ligand in donor-acceptor type bonds. For example, mixing [Cp*Al]4 with the Lewis acidic B(C6F6)3 forms the Al-B donor-acceptor type bond, and results in the synthesis of the adduct [Cp*Al-B(C6F6)3].[14] Analogous main-group complexes that have been synthesised and characterised include dialane complexes [Cp*Al-Al(C6F5)3][15] and [Cp*Al-Al(t-Bu)3],[16] and group 13-group 13 complexes [Cp*Al-Ga(t-Bu)3].[16]
[Cp*Al] is also able to act as a potent ligand to transition metals. For example, treatment of [Cp*Al] with [(dcpe)Pt(H)(CH2t-Bu)] (dcpe = bis(dicyclohexylphosphino)ethane) yields [(dcpe)Pt(Cp*Al)2].[17] Other transition metals which use [Cp*Al] as a ligand include, but are not limited to d10 metal centre complexes such as [Pd(Cp*Al)4] and [Ni(Cp*Al)4],[18] and lanthanide/actinide metal centre complexes such as (CpSiMe3)3U-AlCp*, (CpSiMe3)3Nd-AlCp* and (CpSiMe3)3Ce-AlCp*.[3][19]

References
- ^ a b c d e f g Dohmeier, Carsten; Robl, Christian; Tacke, Matthias; Schnöckel, Hansgeorg (1991). "The Tetrameric Aluminum(I) Compound[{Al(η5-C5Me5)}4]". Angewandte Chemie International Edition in English. 30 (5): 564–565. doi:10.1002/anie.199105641. ISSN 0570-0833.
- ^ a b c Haaland, Arne; Martinsen, Kjell-Gunnar; Shlykov, Sergey A.; Volden, Hans Vidar; Dohmeier, Carsten; Schnoeckel, Hansgeorg (1995). "Molecular Structure of Monomeric (Pentamethylcyclopentadienyl)aluminum(I) by Gas-Phase Electron Diffraction". Organometallics. 14 (6): 3116–3119. doi:10.1021/om00006a065. ISSN 0276-7333.
- ^ a b c d Liu, Yashuai; Li, Jia; Ma, Xiaoli; Yang, Zhi; Roesky, Herbert W. (2018). "The chemistry of aluminum(I) with β-diketiminate ligands and pentamethylcyclopentadienyl-substituents: Synthesis, reactivity and applications". Coordination Chemistry Reviews. 374: 387–415. doi:10.1016/j.ccr.2018.07.004. ISSN 0010-8545. S2CID 105749253.
- ^ a b Schulz, Stephan; Roesky, Herbert W.; Koch, Hans Joachim; Sheldrick, George M.; Stalke, Dietmar; Kuhn, Annja (1993). "A Simple Synthesis of[(Cp*Al)4] and Its Conversion to the Heterocubanes[(Cp*AlSe)4] and[(Cp*AlTe)4](Cp*=η5-C5(CH3)5)". Angewandte Chemie International Edition in English. 32 (12): 1729–1731. doi:10.1002/anie.199317291. ISSN 0570-0833.
- ^ Schormann, Mark; Klimek, Klaus S.; Hatop, Hagen; Varkey, Saji P.; Roesky, Herbert W.; Lehmann, Christopher; Ropken, Cord; Herbst-Irmer, Regine; Noltemeyer, Mathias (2001). "Sodium–Potassium Alloy for the Reduction of Monoalkyl Aluminum(III) Compounds". Journal of Solid State Chemistry. 162 (2): 225–236. Bibcode:2001JSSCh.162..225S. doi:10.1006/jssc.2001.9278. ISSN 0022-4596.
- ^ a b Nagendran, Selvarajan; Roesky, Herbert W. (February 2008). "The Chemistry of Aluminum(I), Silicon(II), and Germanium(II)". Organometallics. 27 (4): 457–492. doi:10.1021/om7007869. ISSN 0276-7333.
- ^ Minasian, Stefan G.; Arnold, John (2008). "Synthesis and reactivity of bis-pentamethylcyclopentadienyl diiododialane (Cp*AlI)2: an aluminium(ii) precursor to (Cp*Al)4". Chemical Communications (34): 4043–5. doi:10.1039/b806804f. ISSN 1359-7345. PMID 18758620.
- ^ Hofmann, Alexander; Lamprecht, Anna; Jiménez-Halla, J. Oscar C.; Tröster, Tobias; Dewhurst, Rian D.; Lenczyk, Carsten; Braunschweig, Holger (2018-08-09). "Lewis-Base-Induced Disproportionation of a Dialane". Chemistry: A European Journal. 24 (45): 11795–11802. doi:10.1002/chem.201802300. ISSN 1521-3765. PMID 29920807. S2CID 49308900.
- ^ a b Himmel, Hans-Jörg; Vollet, Jean (December 2002). "Probing the Reactivity of Aluminum(I) Compounds: The Reaction of Pentamethylcyclopentadienyl-Aluminum, Al[C5(CH3)5], Monomers with Dihydrogen in a Solid Ar Matrix to Give the New Aluminum Hydride Molecule H2Al[C5(CH3)5]". Organometallics. 21 (26): 5972–5977. doi:10.1021/om020787x. ISSN 0276-7333.
- ^ Williams, Kristen S.; Hooper, Joseph P. (2011-12-08). "Structure, Thermodynamics, and Energy Content of Aluminum–Cyclopentadienyl Clusters". The Journal of Physical Chemistry A. 115 (48): 14100–14109. Bibcode:2011JPCA..11514100W. doi:10.1021/jp207292t. hdl:10945/48676. ISSN 1089-5639. PMID 22007955. S2CID 2992445.
- ^ a b Meng, Lingpeng; Zeng, Yanli; Sun, Zheng; Li, Xiaoyan; Lu, Feifei (2015-07-28). "Influences of the substituents on the M–M bonding in Cp4Al4 and Cp2M2X2 (M = B, Al, Ga; Cp = C5H5, X = halogen)". Dalton Transactions. 44 (31): 14092–14100. doi:10.1039/C5DT01901J. ISSN 1477-9234. PMID 26171664.
- ^ Stelzer, Adrian C.; Hrobárik, Peter; Braun, Thomas; Kaupp, Martin; Braun-Cula, Beatrice (2016-04-29). "Completing the Heterocubane Family [Cp*AlE]4 (E = O, S, Se, and Te) by Selective Oxygenation and Sulfuration of [Cp*Al]4: Density Functional Theory Calculations of [Cp*AlE]4 and Reactivity of [Cp*AlO]4 toward Hydrolysis". Inorganic Chemistry. 55 (10): 4915–4923. doi:10.1021/acs.inorgchem.6b00462. ISSN 0020-1669. PMID 27129027.
- ^ Schulz, Stephan; Häming, Ludger; Herbst-Irmer, Regine; Roesky, Herbert W.; Sheldrick, George M. (1994-05-18). "Synthesis and Structure of the First Dimeric Iminoalane Containing an Al2N2 Heterocycle". Angewandte Chemie International Edition in English. 33 (9): 969–970. doi:10.1002/anie.199409691. ISSN 0570-0833.
- ^ Gorden, John D.; Voigt, Andreas; Macdonald, Charles L. B.; Silverman, Joel S.; Cowley, Alan H. (2000). "A Lewis Acid Adduct of an Alanediyl: An Aluminum(I)−Boron Donor−Acceptor Bond". Journal of the American Chemical Society. 122 (5): 950–951. doi:10.1021/ja993537p. ISSN 0002-7863.
- ^ Gorden, John D.; Macdonald, Charles L. B.; Cowley, Alan H. (2001). "A valence isomer of a dialane". Chemical Communications (1): 75–76. doi:10.1039/B007341P. ISSN 1359-7345.
- ^ a b Schulz, Stephan; Kuczkowski, Andreas; Schuchmann, Daniella; Flörke, Ulrich; Nieger, Martin (2006). "Group 13−Group 13 Donor−Acceptor Complexes". Organometallics. 25 (22): 5487–5491. doi:10.1021/om0606946. ISSN 0276-7333.
- ^ Weiss, Dana; Steinke, Tobias; Winter, Manuela; Fischer, Roland A.; Fröhlich, Nikolaus; Uddin, Jamal; Frenking, Gernot (2000). "[(dcpe)Pt(ECp*)2] (E = Al, Ga): Synthesis, Structure, and Bonding Situation of the First Aluminum(I) and Gallium(I) Complexes of Phosphine-Substituted Transition Metal Centers". Organometallics. 19 (22): 4583–4588. doi:10.1021/om000310q. ISSN 0276-7333.
- ^ Buchin, Beatrice; Steinke, Tobias; Gemel, Christian; Cadenbach, Thomas; Fischer, Roland A. (2005). "Synthesis and Characterization of the Novel AlI Compound Al(C5Me4Ph): Comparison of the Coordination Chemistry of Al(C5Me5) and Al(C5Me4Ph) at d10 Metal Centers". Zeitschrift für Anorganische und Allgemeine Chemie. 631 (13–14): 2756–2762. doi:10.1002/zaac.200500129. ISSN 0044-2313.
- ^ Minasian, Stefan G.; Krinsky, Jamin L.; Rinehart, Jeffrey D.; Copping, Roy; Tyliszczak, Tolek; Janousch, Markus; Shuh, David K.; Arnold, John (2009-09-30). "A Comparison of 4f vs 5f Metal–Metal Bonds in (CpSiMe3)3M−ECp* (M = Nd, U; E = Al, Ga; Cp* = C5Me5): Synthesis, Thermodynamics, Magnetism, and Electronic Structure". Journal of the American Chemical Society. 131 (38): 13767–13783. doi:10.1021/ja904565j. ISSN 0002-7863. PMID 19725526.
