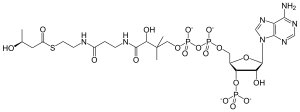
| |
| Names | |
|---|---|
| IUPAC name
{[(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-2-({[hydroxy({hydroxy[(3R)-3-hydroxy-3-({2-[(2-{[(3R)-3-hydroxybutanoyl]sulfanyl}ethyl)carbamoyl]ethyl}carbamoyl)-2,2-dimethylpropoxy]phosphoryl}oxy)phosphoryl]oxy}methyl)oxolan-3-yl]oxy}phosphonic acid
| |
| Identifiers | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| Properties | |
| C25H42N7O18P3S | |
| Molar mass | 853.625 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
β-Hydroxybutyryl-CoA (or 3-hydroxybutyryl-coenzyme A) is an intermediate in the fermentation of butyric acid, and in the metabolism of lysine and tryptophan.[1][2] The L-3-hydroxybutyl-CoA (or (S)-3-hydroxybutanoyl-CoA) enantiomer is also the second to last intermediate in beta oxidation of even-numbered, straight chain, and saturated fatty acids.[3]
See also
References
- ^ Numa, S.; Ishimura, Y.; Nishizuka, Y.; Hayaishi, O. (1961-10-23). "beta-Hydroxybutyryl-CoA, an intermediate in glutarate catabolism". Biochemical and Biophysical Research Communications. 6: 38–43. doi:10.1016/0006-291x(61)90181-4. ISSN 0006-291X. PMID 14480702.
- ^ Liu, Shumeng; Yu, Huajing; Liu, Yongqing; Liu, Xinhua; Zhang, Yu; Bu, Chen; Yuan, Shuai; Chen, Zhe; Xie, Guojia; Li, Wanjin; Xu, Bosen; Yang, Jianguo; He, Lin; Jin, Tong; Xiong, Yundong (2017-09-07). "Chromodomain Protein CDYL Acts as a Crotonyl-CoA Hydratase to Regulate Histone Crotonylation and Spermatogenesis". Molecular Cell. 67 (5): 853–866.e5. doi:10.1016/j.molcel.2017.07.011. ISSN 1097-4164. PMID 28803779.
- ^ van Rijt, Willemijn J.; Van Hove, Johan L. K.; Vaz, Frédéric M.; Havinga, Rick; Allersma, Derk P.; Zijp, Tanja R.; Bedoyan, Jirair K.; Heiner-Fokkema, M. R.; Reijngoud, Dirk-Jan; Geraghty, Michael T.; Wanders, Ronald J. A.; Oosterveer, Maaike H.; Derks, Terry G. J. (July 2021). "Enantiomer-specific pharmacokinetics of D,L-3-hydroxybutyrate: Implications for the treatment of multiple acyl-CoA dehydrogenase deficiency". Journal of Inherited Metabolic Disease. 44 (4): 926–938. doi:10.1002/jimd.12365. ISSN 1573-2665. PMC 8359440. PMID 33543789.
