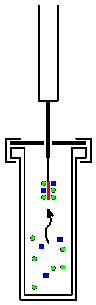
Solid phase microextraction, or SPME, is a solid phase extraction sampling technique that involves the use of a fiber coated with an extracting phase, that can be a liquid (polymer) or a solid (sorbent),[1] which extracts different kinds of analytes (including both volatile and non-volatile) from different kinds of media, that can be in liquid or gas phase.[2] The quantity of analyte extracted by the fibre is proportional to its concentration in the sample as long as equilibrium is reached or, in case of short time pre-equilibrium, with help of convection or agitation.
YouTube Encyclopedic
-
1/1Views:674
-
Field Detection of Odor Signatures 2011 : 03 : Instrumental Detection pt.1
Transcription
[ music ] >> This lecture will cover the instruments that we'll be using during this workshop so we'll go a little bit more into the background of their theory and their uses so here's an outline for the presentation. First I will give differences between trace detection and bulk detection and their advantages and disadvantages, then we will move into the instrument detection technologies and these include Ion Mobility Spectrometry and Gas Chromatography-Differential Mobility spectrometry so during this workshop we'll have use of three different instruments, the first two are by Morpho Detection and these are the Itemizer 3 Enhanced and the Mobile Trace which is actually a hand held IMS so you can take out into the field. For the gas chromatography DMS we'll be using Thermo Scientific's EGIS defender and I will describe a little bit about each instrument so you'll have an idea prior getting into the lab and working with them. Lastly, I'll talk about the operating conditions optimizations that we can do to these instruments in order to enable the detection of the volatile chemical signatures that you just saw the K9 hit on for marijuana so normally these instruments do not come with these compounds configured so they just come with the parent drug and explosives such as RDX or THC programmed into the instrument so through research that we have done at the Amirall lab we've determined how to change the instrumental conditions in order to preferentially detect these compounds. So, the first layer of security in an airport scenario is the metal detector, almost all of us have to go through it, including these penguins, then we also have X-ray systems and here on the top right we see the Vaxus system which we'll later on see a video about and it's scanning these porta potty's at a large venue such as the Super Bowl. Below that we actually see the Vaxus system imaging an actual semi vehicle and we see that they're smuggling human two people are smuggled in that vehicle so it's a very useful tool for the customs folks. We also have computer tomography scanners at the airports that they can visualize the contents of your luggage. So like we just saw trained K9 detection teams are the gold standard for trace detection out on the field and here we see this handler and his K9 searching packages in a warehouse and we also have ion mobility spectrometry, which some of you have experience with. Amplifying florescent polymers and these are polymers that are linked they're chromophores that are linked in a molecular wire when they come into contact with an analyte such as TNT, the florescence's will quench and you can see a response but because they're changed in this molecular wire you can have small amount of analyte produce enough signal so it's kind of analogous to when you have a Christmas light set and one light bulb goes out it usually all the light bulbs go out so that's the same concept with the amplifying florescent polymers and that's why you can get down to the part per trillion levels. Then we also have the color metric kits that show a distinct color change for explosives being present. So for contraband detection we have two different strategies, we can go for trace and bulk detection and these are considered complementary so when we're looking for a trace sample, we're targeting, we're in a scenario where we're trying to show that this person has handled explosives or has become close in proximity to them, we're looking for microscopic amounts so we can't really see these with the naked eye so we need help of a microscope or these very sensitive trace detectors, which are chemical sensors. They're highly specific as compared to the bulk detectors which are just imaging the contents of a container and the bulk detection we're actually looking for the large amount of material that is being obscured, whereas with the trace we're looking for small quantities which could either be the particles or the vapors that are being emitted from a concealed package of explosives or drugs. So bulk detectors use imaging and nuclear properties to show you where the threat could possibly be and here on the bottom we see a current millimeter weight image that is now being used by TSA, where the body parts are not being shown anymore due to the public outcry but it'll highlight the area where the concealment would be. Unfortunately for the bulk detectors they are higher equipment costs but that is mitigated by the fact that if there was an issue happening at a port of entry obviously shutting down that port of entry would be much more costly than investing in these technologies. With trace detection, sampling is very important a very important aspect of trace detection, whereas in bulk you're seeing the whole image of the inside of a container, for trace you have to effectively be able to remove the particles from the luggage or collect that vapor that is being emitted from the drug or explosive so that's what I mean by being very sample dependent. On the top trace you actually see the detection of 240NT by ion mobility spectrometry. So combining trace and bulk detection and having all of these tools in our tool kit help us find that needle in the haystack. So again to reiterate on trace detection we're conducting chemical analysis on very small amounts of contraband material so here we see TNT explosive. Now, like Dr. Almirall was alluding to earlier, we're looking for very small quantities so I made the slide so you guys can visualize what we're talking about when we use these terms such as part per million or parts per billion or nanogram so if you think about what nanogram is, think about a Splenda packet and that weighs 1 gram so imagine we're looking at a billionth of a gram when we're doing these sorts of analyses. And parts per million you can visualize one inch in a 16 mile road or more dilute would be parts per billion which is the second in 32 years. So for trace detectors there are three different steps and the first is collecting your sample, which like I said is very important for trace detection, then separating your different components and analyzing them to produce an alarm and gives the operator an indication that there is possibly a threat agent present. So for collection sample acquisition again is very key, you have to be able to collect that sample whether it be particles or vapors effectively. Now pre-concentration is also important because you might have a minute amount of vapor available in a large volume area of air so you're not going to evacuate all of the air of a room to get that one that small portion of the odor so you need to efficiently be able to pre-concentrate that or screen it down and then once you've collected it you have to have a method of introducing your sample into your instrument. So the next step is actually separating the different compounds that you collect so you take a sample. And you might have 5 to 300 components in there but an efficient trace detector gives you selectivity towards that specific contraband so you can modify instrumental parameters to preferentially produce peaks that are only for your compound that you're searching for and a good trace detector would resolve multiple indicators of contraband so there was a question about if you had a plasticizer and that's ubiquitous in the environment well you would look for that but then you would also have probably another detection channel where you see let's see a volatile component of explosive such as for instance smokeless powders you'll have nitroglycerin which has a high vapor pressure so you'll see that compound along with the other additives to the smokeless powder so that's what I mean by a good trace detector gives you multiple indicators of contraband and I'll also call this channels. And lastly we analyze and the important aspect of trace detection is that it's fast and simple to interpret the results. So here's a point where we actually detect the contraband and trace detectors vary in their sensitivity or the amount of difference you see with concentration differences in your signal and the actual detection limits, or how low can you go, what is the smallest amount that can be detected? So we have a variety of trace samples that we can do that we can analyze with instruments. So first we have to have a source so like we saw earlier with the dog he had a source that contained marijuana and that was emitting volatiles that were coming off of the packet where he had it contained. You can also have particles so where we have TATB particles that are imaged by SCM and below we actually have these COMPS bags they're called Controlled Odor Mimic Permeation Systems and they're simply just a low density polyethylene bag that contains our compounded interest, which in this case is 240NT, which is degradation product of TNT, or an additive in smokeless powders. Now, from studying the weight changes over long periods of time, we can know how much mass is coming off of this bag per second. So that's why we use these COMPS bags for training K9s. So when we present this to the K9 we know ok it's been emitting X nanograms for second so for a given amount of time we know how much odor is available. So it's a good way of training them and having equality in the amount of odor that they're all being trained on and for our purposes we actually use these COMPS devices to calibrate the PSPME IMS system. So again to reiterate particles are the microscopic material of contraband that gets stuck to the surfaces. Now you'll be able to see them when for instance when a bomb maker is touching the bomb and he's not being careful about using gloves or the transfer of particles to the object, the exterior of the object. When we sample for vapors we're looking again for these molecules that are emitted from the solid or the liquid explosive. And we have options in sampling either particles or vapors and on the top we see the analyst swiping a surface and that's considered contact because contact is being made between the swab material and the surface that is suspected of containing the contraband. Then below, that we have non-contact and some of us have been in situations in the airport where we're placed in the people sampler where they puff air all up and down our bodies in order to dislodge any potential particles that you may have from handling drugs during packaging or explosives and those are directed into the analyzer to show that there's presence or not of drugs or explosive. And lastly vapor detection is obviously done with non contact techniques because again it's coming off of the actual drug or explosive. So here's a schematic of the processes that are going on in the ion mobility spectrometer. So again, key is collecting your sample so you have to have it on some sort of medium and you place it into the heated absorber and all of the IMS instruments have this type of desorption unit. And this heat evolves vaporizes here a sample into gas molecules, these molecules are directed into what's called the ionization region and there they're being ionized through a series of chemical reactions and you can even introduce a dopant gas that will selectively ionize certain compounds and prevent others from being ionized themselves. So these ions are gated into the drift region and under a uniform electric field they're propelled towards the detector. Now, separation occurs due to differences in your ions based on shape, size, mass and charge. So, we have this counter flow of drift gas that is coming against the ions that are traveling towards the detector. So if you can visualize it as a man in a crowd trying to go against it, he's gonna have a harder time than a small child running through the crowd, he'll get to his destination faster and that's the principle here and we're just looking at the time of flight from the moment that the ions exit the shutter to the detector and then based on that mobility we can presumptively identify the compounds that we're seeking. So eye mobility spectrometry is used very much in the airport scenario. More than 15,000 instruments conduct over 10 million analyses per year and much of this is due to the fact that the analysis is low cost and the instruments are very easy to operate, which we will see soon. It's very portable, it actually be made into hand held instruments that weigh less than 10 pounds and this is because they undergo the processes that undergo within the ion mobility spectrometer are atmospheric chemical ionization. And that simply just means that we don't need these high vacuum systems, or high purity gases so it makes it very portable. It's very sensitive down to the pecogram. IMS is advantageous because for explosives we obtain very stable negative product ions and for the drugs we obtain from very positive favorable ion response towards these nitrogen containing compounds. And as of late we have dual mode analyzers so we can see our compounds in positive and negative mode so simultaneously you can take one sample and see if it's a drug or an explosive. But with all instrumentation you have disadvantages of course and one of the main ones is that most IMS most commercial IMS systems today include a radioactive source but they're trying to move away from that now. And again, like I eluded to earlier, the detection channels that are programmed into these instruments are based only on the actual parent explosives such as RDX and THC, not the odor signatures that the K9s are alerting to. It's an efficient particle sampler but the vapor sampling needs help because it doesn't have an official sample introduction and collection mechanism and that's what PSPME was designed to do. So here we're going to see a video of the use of an itemizer 3 instrument in a club scene. >> North Whales police are working with partner agencies to protect individuals, their families and communities from the use of illicit drugs. This evening we've deployed the GE ion track itemizer to localize this premises. The device is a desktop drug and explosives detection and identification system. It is capable of detecting traces of a certain number of drugs on people or from any surface. This is part of our overall drug strategy and will assist in identifying and apprehending offenders and detect defenses of possession and supply of control drugs. By doing this we aim to provide a safer environment for members of the public to use license premises while supporting members of the licensing trade and efforts to discourage the use of controlled drugs within their premises. The itemizer enables an objective assessment to be made of the likelihood of an individual having had contact with controlled drugs, not a consequence removes the guesswork from discretionary exercise from police powers. In effect it provides and objective basis on which reasonable grounds can be established for the exercise of police powers. If you have any concerns about drugs in your community, please contact your local policing team or call crime stoppers on 0800-555-1111. If you want free confidential help and advice about drugs, please contact the Whales drug and alcohol help line on 0800-633-5588. >> So you saw that he was conducting particle sampling and he was wiping the hands of the folks at the club but what we want to do is move towards vapor sampling, seeing the odors that are being eminated. So here's another video explaining a little bit more about the IMS systems that we're gonna be using so this is a video concerning the itemizer. >> The itemizer works by heating the sample in the disorber to create vapors that are drawn into the detector and electrically charged by a patented ionization process. At the front of the detector, behind the disorber, is a semi permeable membrane. Narcotics and explosives easily pass through the membrane but inorganic materials and water vapor cannot. Once they pass through the membrane the vapors reach the ionization chamber where they gain or lose electrons and become positively or negatively charged. In the ionization chamber, the vapors react with dopant gases, the dopants are circulated inside the detector by the same pump that draws the sample into the detector. The dopants help to ensure that contraband substances are fully ionized and simultaneously prevent the ionization of other substances that are not targeted for detection. Once the ions are created, an electric field is used to propel them from the ion trap at the front of the detector to the collector electrode at the other end. The current generated by the ions creates peaks that are displayed as a plasma gram. By measuring the time of flight of the ions, substances may be identified. This process allows detection of targeted contraband substances with high sensitivity and selectivity. >> So the first instrument that we'll be using is the itemizer 3E and this one conducts both positive and negative detection so we can simultaneously see drugs and explosives. The analysis is done in as little as 8 seconds and we have the option of doing wipe sampling as you see below or vacuum sampling. But with the vacuum sampling there's no pre-concentration so you're just trying to suck air and hopefully dislodge particles and trap them on that swab material and then you introduce it into the desorber and generally these instruments are very easy to use. The next one is the hand held IMS system from Morpho detection, it's called the Mobile Trace and it weighs only 9.4 pounds. It collects both it detects both particles and vapors but you'll see that with particles you have the nozzle of the disorber that you would have to remove for detection of vapors. But again there's no pre-concentration like PSPME would provide with this instrument. Again, this is also simultaneous dual mode detection so we can see our volatile chemical signatures both in the positive and the negative mode which is advantageous and again the analysis is done in as little as 8 seconds. So differential mobility spectrometry is the next technique that we'll be using and in traditional IMS the mobility is considered constant for certain electric fields but once you increase that electric field beyond the certain point then the mobility of that ion depends more on the strength of the electric field. So the ions you have to find a way to introduce your sample, they're ionized, just as in IMS but there is no shutter on the DMS systems so it's advantageous in that you have 100% duty cycle so you're not pulsing your ions into the detector. You have two plates and an RF volt to just apply to one of them and that RF is perpendicular to the actual gas flow so the ions are going to want to go to either the top electrode or the bottom electrode. But in order but if they go up to the top electrode or the bottom electrode they'll get neutralized so in order to steer the ions through the two parallel plates to the detector you need to introduce a compensation voltage. So during a scan you have many compensation voltages that are its modified so you have a full scan of positive and negative compensation voltages and as it hits the electrometer you'll see this compensation voltage DMS spectrum here you'll see the different species being separated. So separation here is as opposed to IMS is based on the changes in mobility to response to these applied fields. So what Thermo Scientific did was they married gas chromatography, which is a technique used to separate a large number of compounds with the DMS side of it by micro DMS, which is by Cyonex. So the advantages are that with the DMS you get simultaneous positive and negative mode detection but at the same time you're adding a fast pre-separation. So the total analysis time is 16 seconds. And this goes back to the whole idea of resolving multiple threats to give you more of an idea of what's contained in the sample. So you'll have multiple peaks and you can actually identify them based on your DMS output. And currently these instruments are deployed at over 170 airports around the world. So now these volatile chemical signatures that the dogs are honing in on how did we determine that these are the compounds that are being emitted? Well the first step is to conduct head space analysis so we take on a small amount of drug or explosive and we sample the air that is above it. So then we'll introduce that sample into the instrument such as a GMS I mean a GCDMS, sorry a GCMS and we'll obtain identification of these compounds. We can also present the suspected compounds to the dogs that are already trained on drugs so if we have a suspected odor signature for a certain drug we can obtain the dog that is trained on that drug and see if we present that odor signature if they alert to it. And these are the type of studies that have been done at FIU at Dr. Ferton's group and Dr. Almirall's group. So, volatile chemical signatures again are coming off the drugs and explosives and for instance as Dr. Almirall said earlier methyl benzoate a degradation product of cocaine has been proven to be detected by the dogs. For marijuana we have limonene, alphapinene and betapinene that have been shown by head space analysis to be coming off of the marijuana. Also, for RDX we have the option of sampling cyclohexanone and that has a high vapor pressure compared to sorry yeah compared to RDX. So for the smokeless powders we have the volatiles 240 NT, DPA and EC that can be targeted. So again, the commercial instruments are not configured to detect these compounds so we want to take advantage of this knowledge that we have now about which are the compounds that are coming off of the drugs in order to use what we currently have available for detection. So, in order to configure the instruments to detect these compounds, we have to make some changes. So, the ability of us to see these peaks these instruments for these specific compounds depends on the different operating conditions so we can change the dopants to selectively ionize that compound. We can change the drift tube temperature to change the compound from breaking down as it travels through the drift tube. We can change the polarity you know if a certain compound might be seen in the positive mode but not the negative mode. We can also, change that drift flow that impedes movement through the drift tube or increase the sample flow. So you have to find a balance with all of these operating conditions in order to be able to detect a large suite of these volatile chemical signatures. Now, what enabled this work was the development of what is shown here which is the first generation SPME IMS interface. So, I don't know if many of you are familiar with [inaudible] micro extraction but Dr. Almirall will talk a little bit more about that coming up. And it's a pre-concentration technique so we were able to sample the drugs and explosives, collect them on this fiber and introduce them into the IMS and see how we can modify the conditions in order to be able to detect these compounds. So in conclusion: bulk and trace detection are complementary and they're both very important in our kit for detecting drugs and explosives. Trace detection involves particle and vapor sampling. And the detection of vapors that are coming off of the parent drugs and explosives may require changes in instruments that are currently manufactured in their operating conditions. Vapor detection using commercial trace detectors can be improved with pre-concentration and they currently do now have any pre-concentration technique. And that's what we aim to show you here at this work shop the power of the PSPME when it's interfaced with ion mobility spectrometry. And here's some scientific references if you want to go back and refer to some of the work. Do you have any questions? >> So the ethos uses a nickel 63 for ionization? >>[inaudible] >> So it not a chrome discharge. It's radioactive? >> Yes, it's radioactvie. >> Any with the GC front end sometimes there are problems with you know contaminating it or introducing too much compound and saturating it, then you sometimes have to either clean it or replace it. Are there protocols to minimize that from happening that are included with the [inaudible]? >> Well generally you just you want to swipe the sample and sometimes you do get a large hit but the instrument itself has a clean cycle mechanism that puffs air through and actually solvent through to clear out that contamination if it's too much. Okay. Thank you.
Analysis
After extraction, the SPME fiber is transferred to the injection port of separating instruments, such as a gas chromatography and mass spectrometry,[3] where desorption of the analyte takes place and analysis is carried out.
Advantages
The attraction of SPME is that the extraction is fast, simple, can be done usually without solvents, and detection limits can reach parts per trillion (ppt) levels for certain compounds. SPME also has great potential for field applications; on-site sampling can be done even by nonscientists without the need to have gas chromatography-mass spectrometry equipment at each location. When properly stored, samples can be analyzed days later in the laboratory without significant loss of volatiles.[citation needed]
Fiber Coatings
The coating on the SPME fiber can be selected to improve sensitivity for specific analytes of interest; ideally the sorbent layer will have a high affinity for the target analytes.[4][5] There are many commercially available SPME fiber coatings that are combinations of polydimethylsiloxane, divinylbenzene, Carboxen, polyacrylate, and polyethylene glycol.[6][7] However, one downside to many of the commercially available SPME fibers is that they tend to be physically brittle due to their composition.[5] Depending on the characteristics of the target analytes, certain properties of the coating improve extraction such as polarity, thickness, and surface area.[4][8] The sample matrix can also influence the fiber coating selection. Based on the sample and analytes of interest, the fiber may need to tolerate direct immersion as opposed to a headspace extraction.[6]
References
- ^ Spietelun, Agata; Pilarczyk, Michał; Kloskowski, Adam; Namieśnik, Jacek (2010). "Current trends in solid-phase microextraction (SPME) fibre coatings". Chemical Society Reviews. 39 (11): 4524–37. doi:10.1039/c003335a. ISSN 0306-0012. PMID 20882243.
- ^ Mitra, Somenath, ed. (2003). Sample Preparation Techniques in Analytical Chemistry. Wiley-Interscience. p. 113.
- ^ Vas, György; Vékey, Károly (2004). "Solid-phase microextraction: a powerful sample preparation tool prior to mass spectrometric analysis". Journal of Mass Spectrometry. 39 (3): 233–254. Bibcode:2004JMSp...39..233V. doi:10.1002/jms.606. ISSN 1076-5174. PMID 15039931.
- ^ a b Spietelun, Agata; Pilarczyk, Michał; Kloskowski, Adam; Namieśnik, Jacek (2010). "Current trends in solid-phase microextraction (SPME) fibre coatings". Chemical Society Reviews. 39 (11): 4524. doi:10.1039/c003335a. ISSN 0306-0012. PMID 20882243.
- ^ a b Spietelun, Agata; Kloskowski, Adam; Chrzanowski, Wojciech; Namieśnik, Jacek (2012-12-28). "Understanding Solid-Phase Microextraction: Key Factors Influencing the Extraction Process and Trends in Improving the Technique". Chemical Reviews. 113 (3): 1667–1685. doi:10.1021/cr300148j. ISSN 0009-2665. PMID 23273266.
- ^ a b Reyes-Garcés, Nathaly; Gionfriddo, Emanuela; Gómez-Ríos, German Augusto; Alam, Md. Nazmul; Boyacı, Ezel; Bojko, Barbara; Singh, Varoon; Grandy, Jonathan; Pawliszyn, Janusz (2017-12-14). "Advances in Solid Phase Microextraction and Perspective on Future Directions". Analytical Chemistry. 90 (1): 302–360. doi:10.1021/acs.analchem.7b04502. ISSN 0003-2700. PMID 29116756.
- ^ Kumar, Ashwini; Gaurav; Malik, Ashok Kumar; Tewary, Dhananjay Kumar; Singh, Baldev (2008-03-03). "A review on development of solid phase microextraction fibers by sol–gel methods and their applications". Analytica Chimica Acta. 610 (1): 1–14. doi:10.1016/j.aca.2008.01.028. ISSN 0003-2670. PMID 18267134.
- ^ Wardencki, Waldemar; Michulec, Magdalena; Curylo, Janusz (2004-07-29). "A review of theoretical and practical aspects of solid-phase microextraction in food analysis". International Journal of Food Science and Technology. 39 (7): 703–717. doi:10.1111/j.1365-2621.2004.00839.x. ISSN 0950-5423.
Further reading
- Janusz Pawliszyn: Handbook of Solid Phase Microextraction, Chemical Industry Press, 2009.
- Pawliszyn J.: Solid Phase Microextraction: Theory and Practice, Wiley-VCH, 1997.
- Pawliszyn J.: Applications of Solid Phase Microextraction, Royal Society of Chemistry, 1999.
