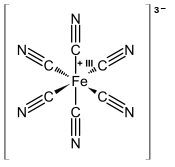
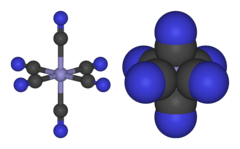
Anion in which a Fe3+ ion is complexed by 6 CN− ions
Chemical compound
Ferricyanide is the anion [Fe(CN)6 ]3− . It is also called hexacyanoferrate(III) and in rare, but systematic nomenclature , hexacyanidoferrate(III). The most common salt of this anion is potassium ferricyanide , a red crystalline material that is used as an oxidant in organic chemistry .[1]
YouTube Encyclopedic
1 / 5
Views: 90 374
760
307
3 808
364
Potassium FerriCyanide: Growing crystals at home
Liquid Sunshine! Bleaching Prints with Ferricyanide (Farmer's Reducer).
How to Write the Formula for Potassium ferricyanide (K3[Fe(CN)6])
Ferrous Sulphate and Potassium Ferricyanide ( Reaction )
Potassium ferricyanide | Wikipedia audio article
Properties [Fe(CN)6 ]3− consists of a Fe3+ center bound in octahedral geometry to six cyanide ligands . The complex has Oh symmetry . The iron is low spin and easily reduced to the related ferrocyanide ion [Fe(CN)6 ]4− , which is a ferrous (Fe2+ ) derivative. This redox couple is reversible and entails no making or breaking of Fe–C bonds:
[Fe(CN)6 ]3− + e− ⇌ [Fe(CN)6 ]4− This redox couple is a standard in electrochemistry .
Compared to main group cyanides like potassium cyanide , ferricyanides are much less toxic because of the strong bond between the cyanide ion (CN− ) and the Fe3+ . They do react with mineral acids, however, to release highly toxic hydrogen cyanide gas.
Uses Treatment of ferricyanide with iron(II) salts affords the brilliant, long-lasting pigment Prussian blue , the traditional color of blueprints .
See also References
^ Gail, E.; Gos, S.; Kulzer, R.; Lorösch, J.; Rubo, A.; Sauer, M.; Kellens, R.; Reddy, J.; Steier, N. "Cyano Compounds, Inorganic". Ullmann's Encyclopedia of Industrial Chemistry doi :10.1002/14356007.a08_159.pub3 . ISBN 978-3527306732
Salts and covalent derivatives of the
cyanide ion
HCN
He
LiCN
<style data-mw-deduplicate="TemplateStyles:r1123817410">'"`UNIQ--templatestyles-00000023-QINU`"'</style><span class="chemf nowrap">Be(CN)<sub class="template-chem2-sub">2</sub></span>
B(CN)<sub>3</sub>
C(CN)4 C2 (CN)2
NH4 CN ONCN O2 NCN N3 CN OCN− -NCO FCN
Ne
NaCN
Mg(CN)2 Al(CN)3 <link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Si(CN)<sub class="template-chem2-sub">4</sub></span>(CH3 )3 SiCN
P(CN)3 SCN− -NCS (SCN)2 S(CN)2 ClCN
Ar
KCN
Ca(CN)2
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Sc(CN)<sub class="template-chem2-sub">3</sub></span>
Ti
V
Cr(CN)6 3−
Mn
Fe(CN)2 Fe(CN)6 4− Fe(CN)6 3−
Co(CN)2 Co(CN)3− 5
Ni(CN)2 Ni(CN)4 2− CuCN
Zn(CN)2 Ga(CN)3 <link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Ge(CN)<sub class="template-chem2-sub">2</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">As(CN)<sub class="template-chem2-sub">3</sub></span>(CH3 )2 AsCN (C6 H5 )2 AsCN
SeCN− BrCN
Kr
RbCN
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Sr(CN)<sub class="template-chem2-sub">2</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Y(CN)<sub class="template-chem2-sub">3</sub></span>
Zr
Nb
Mo(CN)8 4−
Tc
Ru
Rh
Pd(CN)2 AgCN
Cd(CN)2 <link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">In(CN)<sub class="template-chem2-sub">3</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Sn(CN)<sub class="template-chem2-sub">2</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Sb(CN)<sub class="template-chem2-sub">3</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Te(CN)<sub class="template-chem2-sub">2</sub></span>
ICN
Xe
CsCN
Ba(CN)2 *
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Lu(CN)<sub class="template-chem2-sub">3</sub></span>
Hf
Ta
W(CN)<sub>8</sub><sup>4−</sup>
Re
Os
Ir
Pt(CN)4 2- AuCN Au(CN)2 -
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Hg<sub class="template-chem2-sub">2</sub>(CN)<sub class="template-chem2-sub">2</sub></span>Hg(CN)2
TlCN
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Pb(CN)<sub class="template-chem2-sub">2</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Bi(CN)<sub class="template-chem2-sub">3</sub></span>
Po
At
Rn
Fr
Ra
**
Lr
Rf
Db
Sg
Bh
Hs
Mt
Ds
Rg
Cn
Nh
Fl
Mc
Lv
Ts
Og
*
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">La(CN)<sub class="template-chem2-sub">3</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Ce(CN)<sub class="template-chem2-sub">3</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Pr(CN)<sub class="template-chem2-sub">3</sub></span>
Nd
Pm
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Sm(CN)<sub class="template-chem2-sub">3</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Eu(CN)<sub class="template-chem2-sub">3</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Gd(CN)<sub class="template-chem2-sub">3</sub></span>
Tb
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Dy(CN)<sub class="template-chem2-sub">3</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Ho(CN)<sub class="template-chem2-sub">3</sub></span>
Er
Tm
Yb(CN)3
**
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Ac(CN)<sub class="template-chem2-sub">3</sub></span>
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">Th(CN)<sub class="template-chem2-sub">4</sub></span>
Pa
<link href="mw-data:TemplateStyles:r1123817410" rel="mw-deduplicated-inline-style"/><span class="chemf nowrap">UO<sub class="template-chem2-sub">2</sub>(CN)<sub class="template-chem2-sub">2</sub></span>
Np
Pu
Am
Cm
Bk
Cf
Es
Fm
Md
No
This page was last edited on 21 April 2024, at 16:33